On April 6, 2023, Cell Stem Cell published an online research paper entitled "Cynomolgus monkey embryo model captures gastrulation and early pregnancy". This study was conducted in collaboration between the research group led by Dr. Liu Zhen from the CAS Center for Excellence in Brain Science and Intelligence Technology (Institute of Neuroscience), the research team of Dr. Sun Qiang from the Non-Human Primate Research Platform, and the research group of Dr. Zhou Fan from the School of Life Sciences at Tsinghua University. This study focused on establishing an induction system for cynomolgus monkey blastoids using embryonic stem cells with post implantation denelopmental potential of gastrula formation by in vitro culture and triggering early gestational sac's appearance through in vivo transplantation. These significant findings hold great promise for advancing our understanding of primate embryonic development and early pregnancy reactions.
The period spanning embryonic implantation to gastrula movement is a crucial phase in development and also represents a high-incidence period for early clinical abortions. When compared to rodent animal models, the timeline for primate implantation to gastrula movement significantly lags behind. However, embryo-like studies, utilizing embryonic stem cell assemblies, offer a promising avenue for advancing our understanding of early primate implantation development. Currently, mouse embryonic stem cells can be cultured in vitro, progressing to the stages of neurogenesis and organogenesis. Moreover, research has shown that blastocysts induced by human embryonic stem cells can faithfully mimic both the morphological and molecular characteristics of the blastocyst stage. These developments hold substantial potential for enhancing our knowledge of early primate embryonic development.
Non-human primates possess reproductive and embryonic development characteristics that closely resemble those of humans, rendering them an ideal animal model for the study of human reproduction and embryonic development. In a pioneering approach, the research team employed non-human primate embryonic stem cells instead of human pluripotent stem cells to induce cynomolgus monkey blastocysts in vitro. Through a meticulous process that involved comparing cynomolgus monkey embryonic stem cells at different culture states and optimizing the in vitro induction system, the researchers successfully obtained blastoids that exhibited a striking morphological resemblance to cynomolgus monkey blastocysts. This achievement was supported by a comprehensive approach, including single-cell transcriptome sequencing, in-vitro isolation and culture of three lineage stem cells, and a series of other molecular experiments. The results demonstrated that cynomolgus monkey blastoids exhibited similarities in terms of main cell lineage composition and corresponding transcriptome levels when compared to normal blastocysts (Figure 1A-B). To further investigate their in vitro developmental potential, an extended in vitro culture of cynomolgus monkeys was conducted over an extended period.
When subjected to in vitro culture until day 15 (D15), blastoids undergo developmental transitions, culminating in the formation of early implantation bilayer disc-like structures. These structures encompass distinct components, notably the typical OCT4+ epiblast-like ectodermal structure and a prominent amniotic cavity, along with GATA 6+ cells comprising the yolk sac cavity (Figure 1C). Upon further cultivation, it was observed that extending the in vitro culture to D17 results in a subset of cynomolgus monkey embryo double-disc structures continuing to develop. These structures manifest as disc-like formations that feature axis-like structures and cells characteristic of the original chorionic cavity. Some aspects of these structures resemble those reported during the human CS7 (E16-19) developmental stage (Figure 1D). Immunofluorescence staining corroborates these morphological characteristics (Figure 1E). Furthermore, through comprehensive mining and comparative analysis of sequencing data, the authors identified various cell populations within D17 cynomolgus monkey embryos, including epiblast cells (EPI), gastrulating cells (protoblast cells), primordial germ cells (PGCs), amniotic epithelial cells (AME), extra-embryonic mesodermal cells (EXMC), trophoblast cells (TE), and visceral endoderm/yolk sac endodermal cells (VE/YE). Notably, the presence of primitive hematopoietic-related cells, such as hematopoietic endothelial cells (HECs), hematopoietic endothelial progenitor cells (HEPs), and juvenile erythrocytes (Ery), was also detected in D17 cynomolgus monkey embryos. This suggests that D17 cynomolgus monkey embryos have entered the stage of trigermline differentiation.
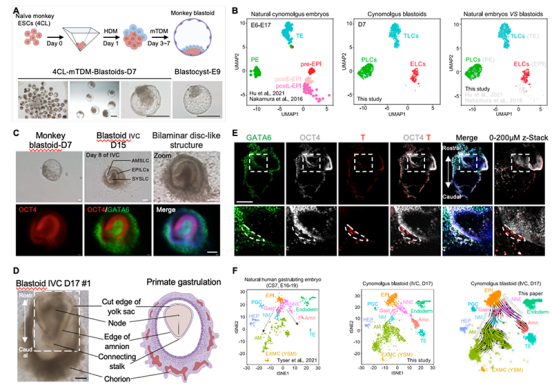
Figure 1. (A) In vitro remodeling of blastocysts from non-human primate cynomolgus monkeys. (B) scRNA-seq analysis of the cell lineage composition of non-human primate cynomolgus monkey blastomeres reconstructed in vitro. (C) Double-layer embryo disc-like structure formed by in vitro delayed culture of non-human primate cynomolgus monkey blastocysts to D15. (D) The gastrula-like structures formed by in vitro delayed culture of nonhuman primate cynomolgus monkey blastoid embryos to D17. (E) Morphological staining of class D17 embryos from non-human primate cynomolgus monkeys. OCT4, EPI marker. GATA6, amine marker. T (Brachyury), gastrulation marker. (F) Single-cell transcriptome analysis and developmental pathway analysis of D17 embryos of non-human primate cynomolgus monkey D 17 class.
The research team conducted further analysis and identified that D17 cynomolgus monkey embryos exhibited populations of cells resembling those found in the embryonic non-neural ectoderm (NNE), neonatal mesoderm (NM), late mesoderm (AM), and stereotypical endoderm (DE) (Figure 1F). This comprehensive examination unveiled that D17 cynomolgus monkey embryos shared similarities in terms of morphology and cell lineage composition with the in vivo human gastrula at the CS7 (E16-19) stage, strongly suggesting that the induced blastoids possess the capacity to replicate post-implantation development. To validate this developmental potential, the team conducted transplantation experiments using cynomolgus monkey blastocysts. The results demonstrated that 3 out of 8 recipients exhibited significant implantation points and the presence of early gestational sacs following transplantation. These findings were accompanied by elevated levels of chorionic gonadotropin and progesterone in the recipients' serum (Figure 2).
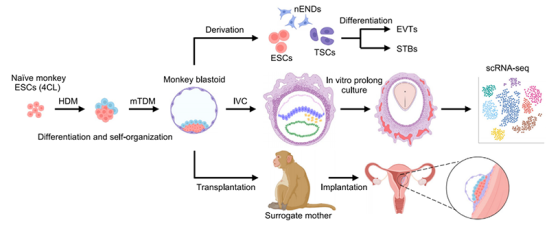
Figure 2. Model of in vitro remodeling and in vitro developmental potential in non-human primates.
In summary, this research marks the first instance of non-human primate blastocysts being cultured in vitro beyond the post-implantation gastrula stage.
Dr. Li Jie (postdoctoral fellow at the CAS Center for Excellence in Brain Science and Intelligence Technology), Zhu Qingyuan (research assistant in the Zhou Fan’s group at Tsinghua University), Cao Jing (doctoral candidate jointly supervised by the CAS Center for Excellence in Brain Science and Intelligence Technology and Northwest Agriculture and Forestry University), Liu Ying (doctoral candidate at Tsinghua University), and Lu Yong (technician at the Non-Human Primate Research Platform of the CAS Center for Excellence in Brain Science and Intelligence Technology) are the co-first authors. Additionally, this work received contributions from other individuals, including Dr. Sun Yidi, Dr. Sun Yining, Huang Yiming, Shang Shenshen, Bian Xinyan, Dr. Li Chunyang, Dr. Zhang Liansheng, Wang Yan, Nie Yanhong, Dr. Fu Jiqiang, and Dr. Li Qian from the CAS Center for Excellence in Brain Science and Intelligence Technology, Li Qian from Tsinghua University, Dr. Miguel A. Esteban, Dr. Li Wenjuan, Dr. Md. Abdul Mazid, Dr. Jiang Yu, and Jia Wenqi from Guangzhou Health Institute of Chinese Academy of Sciences, and Dr. Wang Xiaolong from Northwest Agriculture and Forestry University. This work was supported by the grants from Ministry of Science and Technology, Chinese Academy of Sciences, Shanghai Municipality, SFC and Lingang Laboratory.