- Department:
- Position:Director, Senior Investigator
- Research Field:Electrophysiology, Magnetic Resonance Imaging, Computational neuroscience, Systems neuroscience, Visual perception, Object recognition, Neurovascular coupling, Learning and memory
- Phone:
- E-mail:nikos.logothetis@icpbr.ac.cn
Biographical Sketch
Nikos K. Logothetis is now Co-Director (together with Mu-Ming Poo) of the International Center for Primate Brain Research (ICPBR) in Shanghai, China. Since 1.1.2022 he is also Emeritus Director of the Max Planck Institute for Biological Cybernetics (MPI-BC) in Tübingen, Germany, and Emeritus Adjunct Professor of the Victoria University of Manchester (VUoM), Manchester, UK.
He received a B.S. in mathematics from the University of Athens and a B.S. in biology from the University of Thessaloniki. He studied Piano in the Hellenic Conservatory of Athens and received his Ph.D. in human neurobiology from the Ludwig-Maximilian University in Munich. In 1985 he moved to the Brain and Cognitive Sciences Department of Massachusetts Institute of Technology (MIT), where he initially worked as a postdoctoral fellow and later as Research Scientist.
In 1990, Logothetis joined the faculty of the Division of Neuroscience at the Baylor College of Medicine, and seven years later he was selected as Director in the MPI-BC, where he continued his research in systems neuroscience, including studies of the physiological mechanisms of visual cognition, auditory perception and multisensory integration, as well as investigations of plasticity and neuromodulation in Non-Human-Primates (NHP). Parallel to this ongoing basic research, he has been developing a number of technologies, combining electrophysiology, direct electrical or optical stimulation and pharmacology, with structural and functional Magnetic Resonance Imaging (fMRI). This entirely novel multidisciplinary methodology permitted concurrent investigations of single neurons, microcircuits and neural circuits in NHP.
In addition to his primary affiliations in Germany and UK, since 1992 Logothetis has been Adjunct Professor of Neurobiology at the Salk Institute in San Diego, since 1995 Adjunct Professor of Ophthalmology at the Baylor College of Medicine, Houston, Associate of the Neurosciences Institute, San Diego, Senior Visiting Fellow in University College, London, Adjunct Professor in the Department of Cognitive and Neural Systems at the Boston University, Massachusetts, and as of 2018 Adjunct Professor in the Medical University in Athens, Greece. He has also been elected Visiting Professor of Neuroscience at Stanford University, USA.
He is recipient of several Awards, including the 1996 DeBakey Award for Excellence in Science, the 1999 Golden Brain Award of the Minerva Foundation, the 2003 Louis-Jeantet Prize of Medicine, the 2004 Zülch-Prize for Neuroscience, the 2007 IPSEN Prize for Neuronal Plasticity, the 2008 Alden Spencer Award of Columbia University, New York, and the 2016 Aristeion-Award of the Academy of Athens. He was elected as a member of various Academies, including the German National Academy of Sciences Leopoldina, of the Rodin Remediation Academy, Honorary Member of the American Academy of Arts and Sciences, and a Foreign Associate of the National Academy of Sciences of the United States.
He has been a member of the Advisory Boards of McGovern Institute, Massachusetts Institute of Technology (MIT), in the USA.; Brain and Cognitive Sciences, MIT.; Centre of Excellence in Systems Neuroscience of the Academy of Finland, Helsinki, Finland; Brain Imaging Center, Frankfurt am Main, Germany; ICM-ADREC, Paris, France; Brain Center of the Hebrew University, Jerusalem, Israel; Brain Research Center of the Weizmann Institute, Jerusalem, Israel; and of the advisory board of Institute of Neuroscience, Chinese Academy of Sciences (CAS), and CAS Center for Excellence in Brain Science and Intelligence Technology (CEBSIT), in Shanghai, China.
He served as Receiving Editor for the European Journal of Neuroscience (EJN), Associate Editor for Trends in Cognitive Sciences (TICS), Neuron, Current Biology, Current Opinion Neurobiology, and is a regular reviewer for Nature, Nature Neuroscience, J Neuroscience, PNAS, Cerebral Cortex, Cerebral Blood Flow and Metabolism, Journal of Neurophysiology, Experimental Brain Research, and Vision Research.
He is a member of the Society for Neuroscience (USA), European Neuroscience Association, American Association for the Advancement of Science, Association for Research in Vision and Ophthalmology, New York Academy of Sciences, Society for Industrial and Applied Mathematics, American Mathematical Society, International Neuropsychological Society, and Mathematical Association of America.
Honorsand Prizes
2019 Distinguished Honorary Prof and Doctorate Award, National and Kapodistrian U. of Athens, Greece
2016 The Academy of Athens Award, Athens, Greece
2014 Inaugural Rodolfo Llinás Lecture, New York, USA
2013 Plenary Lecture, Simons Foundation, NY, USA
2012 Special Lecture, SfN, New Orleans, USA
2012 Plenary Lecture, SNL, San Sebastian, Spain
2012 Plenary Lecture, Society of Biological Psychiatry, Philadelphia, USA
2011 Special S.J. Carlson Lecture, Chicago University, Chicago, USA
2011 Julesz Inaugural Lecture, Rutgers University, NJ, USA
2011 Ruth Broad Lecture, Duke University, Durham, USA
2009 Foreign Associate of the National Academy of Sciences, Washington, DC, USA
2008 Alden Spencer Award, Center for Neurobiology and Behavior, Columbia U., New York, USA
2008 Foreign Honorary Member of the American Academy of Arts and Sciences, Cambridge, USA
2007 Annual Adrian Lecture in Neuroscience, Cambridge, UK
2007 The Foundation IPSEN Neuronal Plasticity Prize, (at the IBRO), Melbourne, Australia
2006 Sherrington Centenary Lecture, Oxford University
2006 Annual keynote Body-Mind Lecture. Copenhagen University
2005 Member of the German National Academy of Sciences Leopoldina
2005 Grass Lecture, Halifax, Canada
2005 Plenary Lecture, Japan Neuroscience Meeting
2004 Joachim H. Zülch Prize
2004 Thomas Willis Lecture, Montréal Neurological Institute
2004 FENS Presidential Lecture, Lisbonne, Portugal
2004 Académie des Sciences de l'Institut de France, Paris, France
2004 Plenary Lecture, Sao Paolo, Brazil
2004 Plenary Lecture, The British Psychological Society, London, UK
2003 Louis-Jeantet Prize in Medicine
2003 SFN Presidential Lecture (Pfizer) New Orleans, USA
2002 F.C. Donders Lecture, Nijmegen, NL
2001 Plenary Lecture (Organization for Human Brain Mapping, OHBM), Brighton, UK
2000 Plenary Lecture OHBM, San Antonio, USA
1999 INS Plenary Lecture, Jerusalem, Israel
1999 Golden Brain Award of the Minerva Foundation
1996 Recipient of the DeBakey Award for Excellence in Science
1994 McKnight award
1987 Fairchild fellowship
1981 German DAAD fellowship for Graduate Studies
Alumniof the Logothetis Department in the Period of 2000 to 2021
The following postdoctoral trainees, at the MPI-BC, are currently successful research-scientists, the majority being professors at universities in Europe, Russia, China and United States.
1. Alexander Ecker - Georg-August-Uni Göttingen
2. Alexander Maier - Vanderbilt University, Nashville, TN, USA
3. Amir Shmuel - MNI, McGill University, Montreal, Canada
4. Andreas Bartels - Centre for Integrative Neuroscience, Uni Tübingen, Germany
5. Andreas Tolias - Baylor College of Medicine, Houston, TX, USA
6. Asif Ghazanfar - Princeton University, NJ, USA
7. Bruno Weber - Swiss Federal Institute of Technology, Zurich, Switzerland
8. Christoph Kayser - University of Glasgow, Glasgow, UK
9. Christos Konstantinos - Case Western Reserve University,
10. Chris Petkov - Newcastle University Medical School, Newcastle upon Tyne, UK
11. Christoph Juchem – Yale University, NY
12. David Leopold - National Institute of Mental Health, Bethesda, MD, USA
13. David Omer - Hebrew Uni Jerusalem
14. David Sheinberg - Brown University, Providence, RI, USA
15. George Keliris – University of Antwerp
16. Gregor Rainer - University of Fribourg, Fribourg, Switzerland
17. Goran Angelovski - ICPBR, Shanghai
18. Henry Evrard - INYU, USA - ICPBR, Shanghai
19. Hualou Liang - Drexel University, Philadelphia, PA USA
20. Igor Bondar - Russian Academy of Sciences, Moscow, Russia
21. Jason Kerr (MPG-NKL-Group Leader) – Research Center Caesar (MPG), Bonn
22. Jozien Goense – University of Glasgow
23. Juan Li - Ningbo Institute of Materials Technology & Engineering, CAS
24. Kari Hoffmann - York University, Toronto, Canada
25. Kevin Whittingstall - Université de Sherbrook, Sherbrook, Québec, Canada
26. Kristina Nielsen - John Hopkins, Baltimore, ISA (Starting 2012)
27. Kristine Krug - Chair in Sensory Physiology, Magdeburg
28. Kostas Moutoussis - Athens University, Athens, Greece
29. Masataka Watanabe - Tokyo University
30. Melanie Wilke - University of Göttingen, Göttingen
31. Michael Lippert - Head of Neuroptics
32. Michael Silver - University of California, Berkeley –School of Optometry
33. Michael Schmid – Newcastle University
34. Natasha Sigala - Brighton=Sussex Med School
35. Nelson Totah - Assistant Professor, Helsinki Institute of Life Science
36. Oxana Eshchenko – MPI Biological Cybernetics
37. Paulo Ribeiro -Maranhão Università Brazil
38. Peter Tse - Dartmouth College, Moore Hall, NH, USA
39. Santiago Canals - CSIC, Universidad de Alicante, Spain
40. Stelios Smirnakis - Baylor College of Medicine, Houston, TX, USA
41. Theofanis Panayiotopoulos – University of Leicester
42. Vishal Kapoor - ICPBR, Shanghai
43. Wendy Huddleston - University of Wisconsin, Milwaukee, WI, USA
44. Xiaozhe Zhang - Dalian Institute of Chemical Physics, Chinese Academy of Sciences
45. Zoe Kourtzi – University of Cambridge, UK
Research Impact (Tuesday, August 29, 2023)
Publications 1062
Citations 70224
h-index 122
i10-index 367
Top-10 Publications (Google Scholar; Tuesday, August 29, 2023)
Neurophysiological investigation of the basis of the fMRI signal
NK Logothetis, J Pauls, M Augath, T Trinath… - nature, 2001 - nature.com
Functional magnetic resonance imaging (fMRI) is widely used to study the operational organization of the human brain, but the exact relationship between the measured fMRI signal …
Save Cite Cited by 7666 Related articles All 45 versions
What we can do and what we cannot do with fMRI
NK Logothetis - Nature, 2008 - nature.com
Functional magnetic resonance imaging (fMRI) is currently the mainstay of neuroimaging in cognitive neuroscience. Advances in scanner technology, image acquisition protocols, …
Save Cite Cited by 4059 Related articles All 34 versions
Visual competition
R Blake, NK Logothetis - Nature Reviews Neuroscience, 2002 - nature.com
Binocular rivalry — the alternations in perception that occur when different images are presented to the two eyes — has been the subject of intensive investigation for more than 160 …
Save Cite Cited by 1619 Related articles All 33 versions
Visual object recognition
NK Logothetis, DL Sheinberg - Annual review of neuroscience, 1996 - annualreviews.org
… The neurons found in the temporal lobe of the expert monkeys bear interesting similarities to face-selective cells found in the banks of the rostral STS (NK Logothetis & DL Sheinberg, …
Save Cite Cited by 1385 Related articles All 9 versions
Interpreting the BOLD signal
NK Logothetis, BA Wandell - Annu. Rev. Physiol., 2004 - annualreviews.org
Abstract The development of functional magnetic resonance imaging (fMRI) has brought together a broad community of scientists interested in measuring the neural basis of the …
Save Cite Cited by 1958 Related articles All 14 versions
The underpinnings of the BOLD functional magnetic resonance imaging signal
NK Logothetis - Journal of Neuroscience, 2003 The good coverage and high resolution afforded by functional magnetic resonance imaging (fMRI) make it an excellent tool for the noninvasive imaging of the human brain. Equally …
Save Cite Cited by 1255 Related articles All 18 versions
Shape representation in the inferior temporal cortex of monkeys
NK Logothetis, J Pauls, T Poggio - Current biology, 1995 - Elsevier
Background: The inferior temporal cortex (IT) of the monkey has long been known to play an essential role in visual object recognition. Damage to this area results in severe deficits in …
Save Cite Cited by 1228 Related articles All 20 versions
Multistable phenomena: changing views in perception
DA Leopold, NK Logothetis - Trends in cognitive sciences, 1999 - Elsevier
Traditional explanations of multistable visual phenomena (eg ambiguous figures, perceptual rivalry) suggest that the basis for spontaneous reversals in perception lies in antagonistic …
Save Cite Cited by 1184 Related articles All 19 versions
The neural basis of the blood–oxygen–level–dependent functional magnetic resonance imaging signal
NK Logothetis - … Transactions of the Royal Society of …, 2002 - royalsocietypublishing.org
Magnetic resonance imaging (MRI) has rapidly become an important tool in clinical medicine and biological research. Its functional variant (functional magnetic resonance imaging; fMRI…
Save Cite Cited by 1173 Related articles All 17 versions
Activity changes in early visual cortex reflect monkeys' percepts during binocular rivalry
DA Leopold, NK Logothetis - Nature, 1996 - nature.com
WHEN the two eyes view dissimilar images, we experience binocular rivalry, in which one eye's view dominates for several seconds and is then replaced by that of the other eye 1,2 . …
Synoptic Research Overview
Research is mainly carried out in nonhuman primates (NHP) and occasionally in rodents, the latter mostly for the optimization of methodologies. The biophysical properties of single neurons and microcircuits of small neuronal populations can be studied in any animal, and such studies significantly increase our knowledge of microprocesses. But to understand the primate brain systems and eventually to gain insights into human behavior and its disorders there is no substitute for research in NHP, which has almost the same basic macro-connectivity patterns and cortical organization as humans.
Importantly, for system-studies we dearly need intensive, multidisciplinary and multiscale approaches, to investigate and understand how brain-structures communicate with each other, we need to understand and generatively define so-called “network states” (complete ignorance right now); to comprehend their sequences that may be responsible for certain cognitive capacities and how such states and their orders can warrant causality between brain-activity and cognition; and so on and so forth. Only if we start fathoming into such matters we may eventually reach a point of better understanding malfunctions of the system as well.
Evidently, all of the above require (a) multimodal methods, (b) data-analysis strategies that take into account the multidimensionality of data sets and the different “nature” of the signals (e.g. spiking, field potentials, imaging signals, transfer functions among them, etc.), (c) transfer and further development of mathematical models used in physics for complex dynamic systems into the highly adaptive and nested biological systems, and eventually (d) development and verification of theories that capture the essence of brain networks and their dynamics. About 15 years ago, Paul Thagard, a Canadian philosopher, specialized in cognitive science stated: “Experiment without theory is blind, but theory without experiment is empty”. Yes, indeed. If we want to understand the function of the brain, we must combine experimental work with a variety of theoretical and computational methods, such as accumulator-models, diffusion-models, renewal-models, polynomial state-space models with multiple neural inputs and single, multiple or merged fMRI signals.
Having the aforementioned facts in mind, over the last two decades we have been working intensively for developing and refining an internationally highly acclaimed methodology that permits just that: concurrent intracranial recording of local neural activity and functional Magnetic Resonance Imaging (fMRI) of the entire brain of animals. The application of this method has made significant contributions, amongst other things, to a better understanding of fMRI itself. Non-invasive imaging methods, such as fMRI, can only measure surrogates of neural function, e.g. local metabolic changes in tissues. For this reason, it is vital that we comprehend the neural processes underlying such metabolic changes in order to be able to correctly interpret the functional scans used to assess the condition of patients with various neurological or psychiatric diseases. Beyond the methodological developments, their laboratory made novel and essential contributions in the study of neural mechanisms of conscious visual perception, object recognition, memory and memory consolidation.
The multidisciplinary research, very briefly described above, will dominate our work in the International Center for Primate Brain Research (ICPBR), in Shanghai, for that matter with the hope that we would both continue research in cognitive neuroscience, as well as further develop methodologies, combining multi-scale neurophysiology, neurochemistry, and functional MRI, altogether increasing both our understanding of network activity and the probability of developing methods for envisaging local mesoscopic neural events via the multistructure activity measured with imaging. What follows describes some of the research topics that will continue in the ICPBR of Shanghai, for the years to come.
Neural Mechanisms Underlying Generative Perception & Recognition
First Studies of Multistable Visual perception
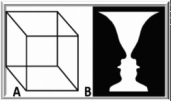 The human brain, although renowned for its awesome computational powers, lapses into profound confusion when it receives conflicting views of the visual world. Consider, for example, the so-called Ambiguous Figures presented in the Figure left (Neckar Cube and Vase-and-Faces). The optical sensory input to the visual system remains unchanged, and yet the resulting perceptual interpretation vacillates over time between alternative views — a behavior called ‘perceptual bistability’. These fluctuations presumably occur because the brain is receiving ambiguous information about the nature of an object at a given location in visual space. Faced with such ambiguity, the brain fluctuates between different neural states over time (Blake & Logothetis, 2002; Leopold & Logothetis, 1999; Logothetis, 1998).
The human brain, although renowned for its awesome computational powers, lapses into profound confusion when it receives conflicting views of the visual world. Consider, for example, the so-called Ambiguous Figures presented in the Figure left (Neckar Cube and Vase-and-Faces). The optical sensory input to the visual system remains unchanged, and yet the resulting perceptual interpretation vacillates over time between alternative views — a behavior called ‘perceptual bistability’. These fluctuations presumably occur because the brain is receiving ambiguous information about the nature of an object at a given location in visual space. Faced with such ambiguity, the brain fluctuates between different neural states over time (Blake & Logothetis, 2002; Leopold & Logothetis, 1999; Logothetis, 1998).
The initial research-aim of Logothetis has been to study and understand what kind of neuronal activity-changes underlie such a perceptual multistability. He was and continues to be strongly interested in this, as he believed that this is not just a quirk of our visual system. Instead, he alleged that it tells us something about so-called Generative Perception, i.e. the bidirectional hierarchical organization of the entire brain and its way of making us aware of sensory information, most often based on experience. To make the perceptual task easy for the NHP, he decided to study the alternations of perception between two dichoptically presented visual stimuli; each in one eye, a phenomenon called binocular rivalry (BR) (Logothetis, 1998).
Until the moment Logothetis started this research, in 1988, the prevailing theory about BR was that it is strictly a “binocular phenomenon” that optimizes unified stereoscopic vision and is utterly unrelated to other multistable perceptual phenomena. Correspondingly, the site of perceptual suppression was thought to be in the primary visual cortex, instantiated in the strong mutual inhibition between orientation-selective cells, e.g. see representative review (Blake, 1989). A few investigators, including Helmholtz, suggested that BR may be related to attention, but then many others used various psychophysical paradigms to further support the quasi-peripheral origin (i.e. involving primary rather than higher association cortices or cortico-thalamo-cortical loops) of this phenomenon. In fact, the belief at that time was that information about the stimulus is entirely blocked after the input layers of striate cortex (V1), and thus is not available to other extrastriate areas such as V2, V4 or MT. Because neurons in the striate sublayers 4Ca and 4B are orientation- and direction-selective, and more than half of the cells in layers 4B and 4A are binocular, undiminished activity in layer 4 should be sufficient for generating the orientation and direction adaptation aftereffects, as well as their interocular transfer reported in a series of psychophysical studies.
Not surprisingly his Science publication (Logothetis & Schall, 1989) was (a) the very first study correlating perception (rather than sensation) with neurophysiology in monkeys and (b) the very first study that provided evidence that neuronal activity in the association visual cortex reflects the perceptual alternations reported by an animal experiencing binocular rivalry; importantly, with solid evidence that the monkey actually performs its task, just like a human would do. Logothetis studied many visual areas in monkeys, and found that the involvement-fraction of single neurons in perception increases as one moves from the primary visual cortex (V1) (10%) to early extrastriate areas (40%) and the temporal visual cortex (90%). For that matter, the neurons in the temporal cortex are entirely silent when the presented stimulus is perceptually suppressed.
An interesting issue that has sparked a great deal of discussion has been the apparent discrepancy between physiological findings in animals and MR neuroimaging results in humans, even though both species reliably report perceptual alternations during rivalry. As mentioned above, while the majority of V1 neurons were not modulated by perceptual switches during binocular rivalry, BOLD fMRI activity in V1 of humans was found to change during the perceptual alternations just as strongly as it does during physical alternation of the visual stimuli (Cerf-Ducastel et al., 2001). Such seeming discrepancies, however, often reflect poor discrimination between attention and awareness (Watanabe et al., 2011). Using a two-by-two factorial functional magnetic resonance imaging design with binocular suppression, it was demonstrated that the visibility or invisibility of a visual target led to only non-significant BOLD effects in the human primary visual cortex (V1). Directing attention toward and away from the target had much greater and more robust effects across all study participants. The difference in the lower-level limit of BOLD activation between attention and awareness illustrates the dissociated neural correlates of the two processes. These results agree with previously reported V1 BOLD effects on attention, at the same time inviting a reconsideration of the functional role of V1 in visual awareness.
Yet, another major debate about the neural correlates of conscious perception concerned its cortical structure-function organization, namely, whether it includes the prefrontal cortex (PFC), which mediates executive functions, or it is constrained within posterior cortices. It has been suggested that PFC activity during paradigms investigating conscious perception is conflated with post-perceptual processes associated with reporting the contents of consciousness or feedforward signals originating from exogenous stimulus manipulations and relayed via posterior cortical areas.
Population Signals, States, and Perceptual Switches
For long time, the study of perception has continued by recording data in the prefrontal and parietal cortex using multi-electrode arrays, which permit the examination of emergent spatiotemporal patterns of neural activity in each area (Panagiotaropoulos et al., 2014; Panagiotaropoulos et al., 2013; Safavi et al., 2014). In one of the studies, a unified neuronal competition model was used to study the dynamics of adaptation and noise processes in binocular flash suppression (BFS), a form of externally induced perceptual suppression, and compare it with the dynamics of intrinsically driven alternations in binocular rivalry (BR). The study demonstrated that the mean population discharge pattern of a perceptually modulated neuronal population detected in electrophysiological recordings in the lateral prefrontal cortex (LPFC) during BFS constrains the dynamical range of externally induced perceptual transitions to a region around the bifurcation separating a noise-driven attractor regime from an adaptation-driven oscillatory regime (Panagiotaropoulos et al., 2013).
The multi-electrode recordings of LFPs used in the aforementioned studies also provide the opportunity to investigate the spatiotemporal organization of neural activity on the scale of several millimeters. In particular, the phases of oscillatory LFPs allow studying the coordination of neural oscillations in time and space and to tie it to cognitive processing. Given the computational roles of LFP phases, it is important to know how they relate to the phases of the underlying current source densities (CSDs) that generate them. By using a volume-conductor model to characterize discrepancies between LFP and CSD phase patterns, the group working on perception unveiled the source of discrepancies between such signals, which are critical for understanding local-global interactions (Hindriks et al., 2016).
Interactions between Visual Cortical Areas
A major debate about the neural correlates of conscious perception concerns its cortical organization, namely, whether it includes the prefrontal cortex (PFC), which mediates executive functions, or it is constrained within posterior cortices. It has been suggested that PFC activity during paradigms investigating conscious perception is conflated with post-perceptual processes associated with reporting the contents of consciousness or feedforward signals originating from exogenous stimulus manipulations and relayed via posterior cortical areas.
This debate was recently addressed by employing an interesting “non-report” paradigm of binocular motion rivalry, where the instantaneous perceptual content as well as spontaneous transitions in this content could be simply inferred from eye movements (e.g. optokinetic nystagmus), rather than via the active pressing of levers, corresponding to the perception of one or the other stimulus (Kapoor et al., 2022).
The results demonstrated that feature-selective prefrontal neurons are modulated concomitantly with subjective perception and perceptual suppression of their preferred stimulus during both externally induced and internally generated changes in conscious perception. Importantly, this enables reliable single-trial, population decoding of conscious contents. Control experiments confirm significant decoding of stimulus contents, even when oculomotor responses, used for inferring perception, are suppressed. These findings suggest that internally generated changes in the contents of conscious visual perception are reliably reflected within the activity of prefrontal populations in the absence of volitional reports or changes in sensory input (Kapoor et al., 2022).
Neural Underpinnings of Visual Object Recognition
Visual cognition does not occur as the tabula rasa. Even newborns come into the world with biases that point them along the path of learning about the faces and places surrounding them. One of the most constructive processes in visual cognition is object recognition, since our three-dimensional understanding of the objects around us are known to us only via brief, often occluded, two-dimensional blips somewhere on our retina. The rest of the process is up to our brains, and will be based on a foundation of extensive visual experience. In other words, cognitive capacities, such as perception, recognition, and learning have a generative nature.
The diversity of tasks that any biological recognition system must solve suggests that object recognition is not a single, general-purpose process. For that matter, evidence from the fields of psychology, neuropsychology, and neurophysiology supports the idea that there are multiple recognition-systems. Data from normal adults, infants, animals, and brain-damaged patients reveal a major distinction between the classification of objects at a basic category level and the identification of individual objects from a homogeneous object class. In addition, psychophysical and neurophysiological studies indicate that one system may represent objects by combinations of multiple views, or aspects, and another may represent objects by structural primitives and their spatial interrelationships.(Hoffman & Logothetis, 2009; Logothetis, 2000; Logothetis & Sheinberg, 1996b).
Striking in primates is the recognition of faces and facial expressions. Face-specific processing in humans was first demonstrated in clinical research. Prosopagnosia is a face-specific agnosia rendering human Patients incapable of recognizing the faces of familiar or famous persons, but sparing their ability to recognize common objects (Logothetis, 2000). The monkey face-recognition system is remarkably similar to that of humans. It is not surprising, therefore, that a great deal of neural tissue is devoted to the processing of facial information in this species, too.
The recognition-related cortical pathway of NHP originates in the primary visual cortex and stretches through the extrastriate areas V2 and V4 to the temporal cortices. In this pathway, the hierarchically highest association area that is exclusively visual is the inferior temporal cortex (IT). It is in this area, where so-called face cells were discovered by Charles Gross at the beginning of the 1970s (Gross et al., 1969; Gross et al., 1972). In their seminal studies the authors reported a few cells that responded best to complex shapes, such as hands, trees, and human and monkey faces, providing the first evidence for a neurophysiological correlate of Konorski’ s gnostic unit (Konorski, 1967). But are faces the only objects represented in this way? It is this question that we have addressed in a series of experiments combining psychophysical and electrophysiological experiments in intensively and optimally trained NHP (Logothetis & Pauls, 1995; Logothetis et al., 1994; Logothetis et al., 1995).
These studies were the very first to present evidence suggesting that at least one aspect of facial processing, the processing of holistic information, may be employed by the primate brain when recognizing any arbitrary homogeneous class of even artificial objects, which the monkey has to individually learn, remember, and recognize again and again from among a large number of distractors sharing a number of common features with 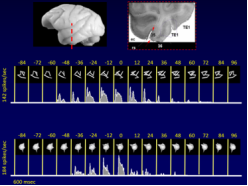 the target (see view-specific neural activity for computer-constructed, artificial objects, such as the wires and amoebas at left). Acquiring such an expertise can induce configurational selectivity in the response of neurons in the visual system. These findings suggested that regarding their neural encoding faces are unlikely to be 'special', but they rather are the default 'special class' of the primate visual system (Logothetis, 2000).
the target (see view-specific neural activity for computer-constructed, artificial objects, such as the wires and amoebas at left). Acquiring such an expertise can induce configurational selectivity in the response of neurons in the visual system. These findings suggested that regarding their neural encoding faces are unlikely to be 'special', but they rather are the default 'special class' of the primate visual system (Logothetis, 2000).
Specifically, most neurons exhibited 3D orientation-dependent responses also during view-plane rotations. Some neurons were found tuned around two views of the same object, while a very small number of cells responded in a view-invariant manner. For five different objects that were extensively used during the training of the animals, and for which behavioral performance became view-independent, multiple cells were found that were tuned around different views of the same object. No selective responses were ever encountered for views that the animal systematically failed to recognize. These results recommended that neurons in this temporal area can develop a complex receptive field organization as a consequence of extensive training in the discrimination and recognition of objects. Simple geometric features did not appear to account for the neurons' selective responses. (Logothetis & Pauls, 1995; Logothetis et al., 1994; Logothetis et al., 1995; Logothetis & Sheinberg, 1996a).
Following the initial results, Logothetis recorded from single neurons while monkeys performed a categorization task with two sets of parametric stimuli. Each stimulus set consisted of four varying features, but only two of the four were important for the categorization task (diagnostic features). Enhanced was selectively the neuronal representation of the diagnostic features, suggesting that stimulus features important for categorization are instantiated in the activity of single units (neurons) in the primate inferior temporal cortex (Sigala & Logothetis, 2002).
Last but not least, one research project concentrated on the neural underpinnings of multisensory perception and recognition. Specifically, the perception of human speech is often enhanced by a combination of auditory and visual signals. Not surprisingly, also animals, and NHP in particular, accompany their vocalizations with distinctive body postures and facial expressions, although it was not known whether the interpretation of these signals is unified. Ghazanfar and Logothetis used a paradigm in which 'preferential looking' was monitored to examine, whether NHP are also able to recognize the correspondence between the auditory and visual components of their calls. The results indicated that rhesus monkeys have an inherent ability to match acoustically presented conspecific vocalizations with the appropriate facial posture. The pattern of the results closely follows those for cross-modal speech recognition by prelinguistic human infants using the same preferential-looking paradigm. Yet this was the first demonstration of auditory–visual integration in an animal vocal communication system (Ghazanfar & Logothetis, 2003).
Neurovascular-System-Identification for Optimal Interpretation & Use of fMRI Signals
The aforementioned multidisciplinary and multiscale methodologies can also greatly help us to develop realistic mathematical models of the fMRI signals, permitting their use – together with the neural signals - for the description of dynamics of brain systems and subsystems.
Yet, the path to study the neurovascular system is anything but straight forward. Well established, classic system identification techniques fall short when dealing with complex ensembles, such as those comprised of neural, glial, and vascular components. More so, when the neurovascular ensemble of some brain structures appears to have strong feedback loops (i.e. vascular activity modulating the neural activity), falling into the category of “non-causal” systems.
With successfully combined neurophysiology and fMRI experiments, one can not only fathom into the neural origin of the up- and down-modulation of metabolic demands, by directly recording the activity of single neurons, microcircuits, and columns, but also estimate Polynomial State-Space Models with multiple neural inputs and a selection of fMRI sub-signals. What follows offers a synoptic description of relevant system-components, the understanding of which may enable the use of multi-structure-activity for localizing and predicting neural activities.
Effects of Cortical Microcircuitson the fMRI Signals
Obviously, correct interpretation of MRI signals requires considering the organizational principles of microcircuits, the cortical ones being a well-studied example. Specifically, Excitation-Inhibition (E-I) microcircuits have certain distinct features: (1) in contrast to the point-neuron, the final response of each real neuron is determined by all feedforward, feedback and modulatory synapses rather than by the linear summation of its inputs; (2) transient excitatory responses result from leading excitation, for example due to small synaptic delays or differences in signal propagation speed, whereupon inhibition is rapidly engaged, followed by balanced activity; (3) net excitation or inhibition might occur when the afferents drive the overall E-I balance in opposite directions; (4) responses to large, sustained input changes may occur while maintaining a well-balanced excitation–inhibition; and (5) the strong recurrence of E-I microcircuits renders them capable of sustained responses (microcircuit memory) to transient stimuli (detailed descriptions and citation can be found in (Logothetis, 2008)).
It follows that changes with balanced E-I are good candidates for mechanisms that adjust the overall excitability and the signal-to-noise ratio of the cortical output, and that depending on their mode of operation, microcircuits can act either as drivers, faithfully transmitting stimulus- or movement-related information, or as modulators that adjust the overall sensitivity and context specificity of the responses.
For studies at the system level, it is important to know whether different types of activity can be identified and measured in a manner permitting at least some dissociation of between driver and feedforward activity, and whether the fine tuning of such processing takes place by means of neuromodulation and feedback. Which aspects can best be studied electrophysiologically, and which require local measurements such as various types of optical imaging?
Our own working hypothesis in many such projects at the microcircuit-level is that various aspects of the extracellular field potential may to some extent reveal different operation modes of E-I microcircuits, which in turn may tell us something about the state of associative networks if information from simultaneous recordings in different areas is combined in one experimental session. A number of observations suggest that the state of E-I cortical microcircuits does indeed influence the global behavior of networks. Network frequency, for example, depends on both synaptic time scales and the balance between excitation and inhibition (for references see (Mazzoni et al., 2008)). Similarly, the multimodal operation of E-I circuits is most likely one of the causes of the brain’s complex pattern of activity, with its extraordinarily rich spatial and temporal structure, a structure or “internal state” that still remains very sensitive to external sensory input.
To address this range of questions, experiments were conducted in various cortical areas, including visual, auditory, somatosensory, and higher associational cortices, and analysis methods were developed in parallel with modeling work (Belitski et al., 2010; Besserve et al., 2010; Ecker et al., 2010; Eschenko et al., 2011; Kayser et al., 2011; Ludtke et al., 2010; Magri et al., 2009; Mazzoni et al., 2010; Panzeri et al., 2010; Rasch et al., 2009). The next step involved examining the validity of these results in other sensory systems, and – most importantly – in the context of behavior. The modulation of individual, non-redundant frequency ranges, the predictability of different types of activity (e.g. spiking from various LFP bands), and the dissociation of various signals in different behavioral contexts may eventually begin to offer insights into mechanisms rather than report correlations between microscopic activity and behavior.
An important issue when investigating microcircuit activity is the amount of information that might be encoded by such structures, given their dense micro-connectivity. Functional, dynamic connectivity in such microcircuits has often been thought to determine the amount of correlated trial-to-trial variability in the activity of neurons. Numerous studies in the past have reported a high degree of correlated variability between nearby cortical cells. Recently, chronically implanted multielectrode arrays were developed, that offer an unprecedented quality of recording in order to reexamine this question in the primary visual cortex of awake macaques. The findings suggested that even nearby neurons with similar orientation tuning show virtually no correlated variability (Ecker et al., 2010). These findings suggest one of two things: either the adjacent neurons share only a few percent of their inputs or their activity is actively decorrelated. Interestingly, active decorrelation also characterizes certain perceptual states (see below), and it might be one basic condition for maximizing the information transmitted from one microcircuit to the other.
Interpretation of the Up- and Down-Regulation of Metabolic Activity
The Neural-Event-Triggered (NET) fMRI results raise the question of correct interpretation of fMRI signals, which correlate with changes in metabolism rather than neural activity. MR signal interpretation is more complicated during spontaneous activity. Oscillations and synchrony could, in principle, reduce the metabolic requirements by increasing the synaptic efficiency. However, the oscillations and the gamma rhythm are associated with high energy demands that require high oxidative energy metabolism, strong mitochondrial performance, and sufficient supply with oxygen and nutrients (Kann, 2011; Lord et al., 2013).
In general, the variance of positive BOLD responses (PBR) in the cortex is best explained by the dynamics of different band-limited-power signals derived from the LFP in anesthetized or drug-free animals. The interpretation of the LFP itself may also require caution because it reflects both sensory input and neuromodulation-induced changes in the local excitation-inhibition balance (Goense & Logothetis, 2008; Lippert et al., 2010; Logothetis et al., 2001). However, the relationship of neuronal inhibition and BOLD responses is not straightforward. The inhibition may increase or decrease energy consumption depending on the extent of local interneuron involvement (Logothetis, 2008). Certain interneuronal classes directly control blood flow regulation (Buzsaki et al., 2007), and the hemodynamic mechanisms of negative BOLD responses (NBR) are different from those of PBR (Goense et al., 2012). Nonetheless, NBR itself may be seen as a reasonable marker of reduction of population-activity because it is often correlated with decreases in multiunit activity (MUA) (Logothetis, 2008; Shmuel et al., 2006).
Studies of Neurovascular Coupling with Electrophysiological Recordings & fMRI
The first results on neurovascular coupling were published in Nature in 2001. That study, presented the first concurrent intracortical recordings of neural signals and fMRI responses. The findings demonstrated that the fMRI BOLD contrast mechanism reflects the input and intracortical processing of a given area rather than its spiking output. In fact, increases in BOLD responses may occur simultaneously with strong decreases in the firing of projection neurons. This and several other subsequent studies demonstrated that the fMRI signal cannot easily differentiate between function-specific processing and neuromodulation, between bottom-up and top-down signals, and it may potentially confuse excitation and inhibition. The magnitude of the fMRI signal cannot be quantified to accurately reflect differences between brain regions, or between tasks within the same region. The origin of the latter problem is not our current inability to accurately estimate oxygenation from the BOLD signal, but the fact that hemodynamic responses are sensitive to the size of the activated population, which may change as the sparsity of neural representations varies spatially and temporally. In cortical regions in which stimulus- or task-related perceptual or cognitive capacities are sparsely represented (e.g. instantiated in the activity of very small number of neurons), volume transmission, which likely underlies the altered states of motivation, attention, learning, and memory, may dominate hemodynamic responses and make it impossible to deduce the exact role of the area in the task at hand.
Development of Multi-Disciplinary Methods for Understanding Brain Networks
Brain: A Complex Dynamic System Par Excellence
Brains are characterized by a vast number of elements, ultra-high structural complexity, and massive connectivity, all of which change and evolve in response to experience. Information related to sensors and effectors is processed in a both parallel and recurrent hierarchical fashion. The connectivity between different hierarchical levels is bidirectional, and its specificity and effectiveness are continuously controlled by associational and neuromodulatory centers. Typically, any observed brain activity is probabilistic, and its evolution is initial-condition dependent.
In mathematical physics such systems are known as Complex Dynamic Systems (CDS), whereby “Complex” does not mean complicated. Instead it implies that the behavior of the whole is emerging - during the process of self-organization - and it cannot be reduced to, or predicted from, the system’s components.
Complex systems are ubiquitous in nature, ranging from the non-adaptive (naCDS) convection cells, snowflakes, weather and climate patterns, to adaptive ones (aCDS), such as economies, social systems, genome, and surely nervous systems.
Most of the aforementioned examples have long been studied intensively using this CDS approach, and these studies have undoubtedly advanced our ability to predict evolution-paths of “random-looking” system-states. An outstanding example is the currently impressive track of weather and climate changes, both dissipative dynamical systems that possess a global attractor with chaotic dynamics, the prediction of which – not surprisingly – has a finite time horizon (Soldatenko & Yusupov, 2021)
By sharp contrast, the application of CDS in systems neuroscience has been very limited and rather “selective”, mostly encountered in human studies using almost exclusively neuroimaging techniques, such as various forms of functional magnetic resonance imaging (fMRI) (Bullmore & Sporns, 2009; Friston, 2002; Ryali et al., 2016). Yet, neural activity in such cases can only be indirectly estimated, mainly reflecting changes in metabolic energy demands. Such measures cannot differentiate between input/output-specific processing and neuromodulation, between bottom-up and top-down signals, and they may occasionally confuse excitation and inhibition (Logothetis, 2008). Moreover, the information provided by fMRI is mostly at macroscopic scale levels – barring cases of combination of very high-field scanners and electroencephalography (EEG), which may provide some mesoscopic information e.g. (Bandettini et al., 2021) – and currently cannot be used to estimate the underlying local neural activities, and the microscopic self-organization processes.
A multimodal approach in systems neuroscience is now more necessary than ever, particularly for the study of the brain’s function and dysfunction. Such an approach may definitely include further improvements of the MRI technology, but most importantly, should enable its combination with other invasive techniques that directly assess the brain’s electrical and neurochemical activity.
Important is also a profound understanding of the neural basis of the brain-structure-specific Hemodynamic Response Functions (HRF), as well as of the deconvolution models permitting the estimation of neural signals from the fMRI time series. In successfully combined neurophysiology, neurochemistry and fMRI experiments, one cannot only fathom into the neural origin of the up- and down-modulation of metabolic patterns, by directly recording the activity of single neurons, microcircuits, and assemblies, e.g. cortical columns, but one can also use the collected multimodal and multi-scale data for realistically enabling the application of CDS, the latter being the only way for accurately describing brain states, their transitions and sequences, and the relationship of the latter to our cognitive capacities.
Neural Subsystems Related to Learning and Memory
It is a well-known fact that the central function of the brain is to create and retain internal representations of the world that can guide behavior. Expressed simply, memory refers to this “retention”. Yet the world is continuously changing, and strict, rigid retention could be worthless, if not detrimental. Learning, i.e. a continuous adaptation of a representation to a changing environment, is thus an essential complementary brain function. Both learning and memory are system properties that reflect the self-organization of concerted operations of micro-, meso- and macro networks at multiple levels of the brain. On the basis of rich experimental evidence, it has been proposed that learning and memory are instantiated in the cooperative-synergistic functions of at least three subsystems (Delacour, 1999).
One subsystem codes and represents sensory information or motor programs with high precision, and probably consists of neuronal assemblies in primary sensory and motor areas, in association cortices, and in structures such as the striatum and cerebellum.
The second subsystem consists of neuronal assemblies with no precise relationship to sensory inputs or motor outputs, but which reflect internal states of the organism, such as arousal or motivation. This subsystem is thought to include the reticular formation, the raphe nuclei, the locus coeruleus, some thalamic/hypothalamic nuclei, the nucleus basalis (Meynert) of telencephalon, and importantly, the limbic system, consisting of diencephalon and cerebrum components, such as the anterior thalamic and septal nuclei, hypothalamus, mamillary body, cingulate, parahippocampal gyri and hippocampus.
Finally, the third subsystem serves the goal-director character of behavior, potentially including structures such as the prefrontal cortex. This last subsystem is likely instantiated in the concerted and synergistic actions of the first two ones.
Synergistic or antagonistic actions, in and among such subsystems, have been considered to reflect synchronization of various types of short or long-lasting oscillatory activities (Buzsaki et al., 2013; Freeman, 2008), which were studied in great detail in various systems, including the thalamocortical system, basal ganglia, hippocampal formation and the brainstem. Rhythmic activity often echoes the interactions of populations of neurons (Buzsaki, 2002; Sirota & Buzsáki, 2005), albeit in the case of the thalamocortical networks, it may be also generated by single neurons as a result of an interplay between specific intrinsic currents (Steriade & Llinas, 1988).
Waves and Intrinsic Neural Events: Global Probabilistic Indicators of Brain-States
One example of slow oscillatory activities, often termed Slow-Waves, is that involving thalamic and cortical structures (McCormick & Bal, 1997; Steriade et al., 1993). During this slow (0.5-1.5 Hz) oscillation the membrane potential of both excitatory and inhibitory cells alternates between depolarized (up) and hyperpolarized (down) states and these excitability phases and their transitions strongly affect the frequency of occurrence of other cortical (Amzica & Steriade, 1997; Molle et al., 2002) and hippocampal (Battaglia et al., 2004; Molle et al., 2006; Sirota et al., 2003) oscillatory patterns.
Another example is the fast-oscillatory activity observed during the large-amplitude deviations of the hippocampal Local Field Potential (LFP), known as Sharp-Waves. The fast-field oscillations (from 100 to over 300 Hz depending on species), are named Sharp-Wave-Ripples (SWR) (Buzsaki et al., 1992; O'Keefe & Nadel, 1978). SWR are a characteristic example of so-called spontaneous Intrinsic Neural Events (INE).
Interestingly, ripples are release phenomena. During active waking, the hippocampus (HP) is dominated by the theta rhythm. This rhythm is controlled by a network of cells extending from the brainstem to the Medial Septum and Diagonal Band of Broca (MSDB), hippocampus and entorhinal cortex (Buzsaki, 2002). MSDB modulates subsets of hippocampal interneurons and principal cells engendering the local theta rhythm (Buzsaki, 2002). When theta is reduced, the CA3 network exhibits a highly synchronized population of spiking bursts that produce large LFP deflections in the dendrites of CA1 pyramidal cells of stratum radiatum. The massive depolarization of CA1, in turn, induces a short-lived dynamic interaction between the aforementioned cell populations, yielding ripples (Buzsaki et al., 1992). SWR are temporally linked to cortical spindles (Axmacher et al., 2006; Siapas & Wilson, 1998), as well as to slow oscillations, and are considered to be part of a large-scale system of oscillatory networks. The coupling of these networks is thought to coordinate specific information transfer between neocortical and hippocampal cell assemblies (Isomura et al., 2006; Sirota & Buzsáki, 2005; Wierzynski et al., 2009).
An additional extensively studied class of INE, also associated with brainwide activity, are the phasic electric potentials that have been recorded from the pons, lateral geniculate nucleus (LGN), and occipital cortex. These electric potentials constitute a hallmark of rapid-eye-movement (REM) sleep (Datta, 2010). Similar to sharp waves, the Pontine-Geniculate-Occipital waves (PGOw) are short (≈ 100 msec) but relatively large (> 300 µV) LFP deflections. The first detailed description and propagation of the PGOw came from electrophysiological recordings in pons, LGN and the cortex of cats (Bizzi & Brooks, 1963; Brooks & Bizzi, 1963). PGOw are related to several important brain functions including sensorimotor integration, dreaming, development and learning (Datta, 2000). Their relation to large-scale networks has been recently demonstrated also in human fMRI studies reporting REM-related activations in the pontine tegmentum, ventro-posterior thalamus and the primary visual cortex in the absence of any visual input (Miyauchi et al., 2009).
Decades of experimental work in animals and humans suggested that the aforementioned INE-examples reflect state changes of self-organizing large-scale networks. The number of ripples increases after learning and their intensification appears to predict memory recall both in rats (Eschenko et al., 2008; O'Neill et al., 2008) and in humans (Axmacher et al., 2008). Conversely, the elimination of ripples by the electrical stimulation of HP during the post-learning Slow-Wave-Sleep (SWS) – also known as NREM, i.e. Non-REM – interferes with memory consolidation (Ego-Stengel & Wilson, 2010; Girardeau et al., 2009). Similarly, disruption of PGOw and REM-sleep appears to selectively interfere with the retention of procedural knowledge (Bavelier et al., 1998).
Evidently, the exact topologies of such networks and the emerging dynamic activity patterns resulting from their evolution over time are likely to be excellent indicators of specific brain functions and dysfunctions. However, electrophysiological recordings of neural events commonly involve one or more brain regions chosen by means of their anatomical connectivity patterns or by formerly established cooperative interactions of structures in the context of behavior. Global states associated with events remain elusive because of the dearth of methodologies permitting concurrent local recordings and whole-brain activity mapping. Functional MRI, on the other hand, provides complete patterns of spontaneous or stimulus-involved Multi-Structure-Activity (MSA), but without concurrent recordings of local waves or spontaneous intrinsic events. Precise prediction of any events, based on MSA patterns is currently impossible.
Concurrent Recordings of INE & MSA: First System-Insights into Memory Consolidation
There are currently two main hypotheses for the mechanisms underlying the consolidation of memory during sleep. The synaptic homeostasis hypothesis assuming that consolidation is a by-product of the global synaptic downscaling that occurs during sleep, and the active system consolidation hypothesis proposing that an active consolidation process results from selective re-activation of memories during sleep. Yet, the two models are not mutually exclusive; the hypothesized processes probably act in concert to optimize the memory function of sleep. A great amount of data is consistent with the notion of sleep promoting experience-dependent synaptic embossing (Blanco et al., 2015; Calais et al., 2015; Ribeiro, 2012), which is actually understood as the simultaneous non-Hebbian downscaling and Hebbian upscaling of separate but complementary sets of synapses, heterogeneously activated at the time of memory encoding and therefore differentially affected by sleep, e.g. see reviews (Diekelmann & Born, 2010; Dudai et al., 2015).
Less than a decade ago, concurrent electrophysiological recordings and fMRI demonstrated that the SWR events are actually tightly associated with robust cortical activations that occur concurrently with a particularly intriguing strong inhibition of large portions of subcortical brain structures that are closely involved in neural plasticity, such as the basal ganglia (BG), cerebellar cortex, and the pontine region (PONS), i.e. the ascending reticular arousal system, that may be involved in synaptic consolidation (Logothetis, 2015; Logothetis et al., 2012). Strikingly, in primates, the negative BOLD in the pontine region was systematically associated with inhibition of the lateral geniculate nucleus (LGN) and foveal V1 activity, despite the overall positive fMRI responses in peripheral V1 and all other primary sensory and associational cortices. The deactivation of PONS may therefore be due to a temporary suppression of cholinergic sites involved in local plasticity and synaptic consolidation, such as those underlying the generation propagation of theta rhythm PGOw.
If PGO waves are indeed associated with the consolidation of procedural memory or synaptic consolidation as mentioned above, the temporal relationship of PGOw to SWRs and the dynamics of MSA patterns could greatly help us understand the interaction between the processes of system and synaptic consolidation. A recent study, combining again NET-fMRI with multishank and multisite recordings in the hippocampus, thalamus and the region of the parabrachial nucleus (PBn), did indeed demonstrate that the brainstem transiently modulates hippocampal network events through the phasic PGOw (Ramirez-Villegas et al., 2021).
Strikingly, two physiologically distinct types of PGOw were found to occur sequentially, selectively influencing high-frequency ripples and low-frequency theta events, respectively. The two PGOw types were associated with opposite hippocampal spike-field coupling, prompting periods of high neural synchrony of neural populations during periods of ripple and theta instances.
The coupling of PGOw and hippocampal ripples is both novel and surprising. Furthermore, PGOw may co-occur with hippocampal Theta-like bursts during short periods of time just as they do during REM sleep in rodents and cats. Putative mechanisms for such selective neuronal ensemble modulations are twofold: Firstly, cholinergic neuromodulation associated with the ascending brainstem-hippocampus synchronizing pathway that terminates in the medial septum and diagonal band of Brocca, which is known to play a key role in Hippocampal Theta rhythm generation, and second a direct pontine input to the hippocampus proper as has been indicated by anatomical studies.
The observations of the influence of PGOw on hippocampal spike-field coupling recommends a putative mechanism which would explain the dramatic opposite changes in excitability during NREM- and REM-like states (Grosmark et al., 2012). Such complementary mechanisms are likely controlled by a common phenomenon spanning sleep states, namely the PGOw. The episodes, representing brainstem cells’ synchronous depolarizations, may correspond to windows for promoting hippocampal plasticity during NREM-like and REM-like states, and this might occur through sequences of low-frequency-modulated SWR and Theta events. This hypothesis is consistent with studies in vitro (Huerta & Lisman, 1995) and in vivo (Poe et al., 2000), highlighting the importance of studying brain-wide transient mechanisms to understand brain function at a system level.
Lastly, the coupling between PGOw and ripples, which are classically associated with distinctly different sleep stages, supports the notion that a global coordination mechanism of hippocampal sleep dynamics by cholinergic pontine transients may promote systems and synaptic memory consolidation as well as synaptic homeostasis.
Literature
Amzica, F., & Steriade, M. (1997). The K-complex: its slow (< 1-Hz) rhythmicity and relation to delta waves. [see comments]. Neurology, 49(4), 952-959.
Axmacher, N., Elger, C. E., & Fell, J. (2008). Ripples in the medial temporal lobe are relevant for human memory consolidation. Brain, 131, 1806-1817. https://doi.org/10.1093/brain/awn103
Axmacher, N., Mormann, F., Fernandez, G., Elger, C. E., & Fell, J. (2006). Memory formation by neuronal synchronization [Research Support, Non-U.S. Gov't
Review]. Brain Res Rev, 52(1), 170-182. https://doi.org/10.1016/j.brainresrev.2006.01.007
Bandettini, P. A., Huber, L., & Finn, E. S. (2021). Challenges and opportunities of mesoscopic brain mapping with fMRI. Current Opinion in Behavioral Sciences, 40, 189-200. https://doi.org/10.1016/j.cobeha.2021.06.002
Battaglia, F. P., Sutherland, G. R., & McNaughton, B. L. (2004). Hippocampal sharp wave bursts coincide with neocortical "up-state" transitions [Comparative Study Research Support, Non-U.S. Gov't
Research Support, U.S. Gov't, P.H.S.]. Learn Mem, 11(6), 697-704. https://doi.org/10.1101/lm.73504
Bavelier, D., Corina, D., Jezzard, P., Clark, V., Karni, A., Lalwani, A., Rauschecker, J. P., Braun, A., Turner, R., & Neville, H. J. (1998). Hemispheric Specialization for English and ASL-Left Invariance Right Variability. NeuroReport, 9(7), 1537-1542. (IN FILE)
Belitski, A., Panzeri, S., Magri, C., Logothetis, N. K., & Kayser, C. (2010). Sensory information in local field potentials and spikes from visual and auditory cortices: time scales and frequency bands [Article]. Journal of Computational Neuroscience, 29(3), 533-545. https://doi.org/10.1007/s10827-010-0230-y
Besserve, M., Scholkopf, B., Logothetis, N. K., & Panzeri, S. (2010). Causal relationships between frequency bands of extracellular signals in visual cortex revealed by an information theoretic analysis [Article]. Journal of Computational Neuroscience, 29(3), 547-566. https://doi.org/10.1007/s10827-010-0236-5
Bizzi, E., & Brooks, D. C. (1963). Pontine Reticular Formation - Relation to Lateral Geniculate Nucleus During Deep Sleep. Science, 141(357), 270-&.
Blake, R., & Logothetis, N. K. (2002). Visual competition [Review]. Nature Reviews Neuroscience, 3(1), 13-23. https://doi.org/10.1038/nrn701
Blake, R. R. (1989). A Neural Theory of Binocular Rivalry. Psychological Review, 96, 145-167. (NOT IN FILE)
Blanco, W., Pereira, C. M., Cota, V. R., Souza, A. C., Renno-Costa, C., Santos, S., Dias, G., Guerreiro, A. M., Tort, A. B., Neto, A. D., & Ribeiro, S. (2015). Synaptic Homeostasis and Restructuring across the Sleep-Wake Cycle. PLoS Comput Biol, 11(5), e1004241. https://doi.org/10.1371/journal.pcbi.1004241
Brooks, D. C., & Bizzi, E. (1963). Brainstem Electrical Activity During Sleep. Anatomical Record, 145(2), 211-&.
Bullmore, E., & Sporns, O. (2009). Complex brain networks: graph theoretical analysis of structural and functional systems. Nat Rev Neurosci, 10(3), 186-198. https://doi.org/10.1038/nrn2575
Buzsaki, G. (2002). Theta oscillations in the hippocampus. Neuron, 33(3), 325-340. http://www.ncbi.nlm.nih.gov/pubmed/11832222?dopt=Citation
Buzsaki, G., Horvath, Z., Urioste, R., Hetke, J., & Wise, K. (1992). High-frequency network oscillation in the hippocampus. Science, 256(5059), 1025-1027.
Buzsaki, G., Kaila, K., & Raichle, M. (2007). Inhibition and brain work. Neuron, 56(5), 771-783. https://doi.org/S0896-6273(07)00927-0 [pii]
10.1016/j.neuron.2007.11.008
Buzsaki, G., Logothetis, N., & Singer, W. (2013). Scaling brain size, keeping timing: evolutionary preservation of brain rhythms. Neuron, 80(3), 751-764. https://doi.org/10.1016/j.neuron.2013.10.002
Calais, J. B., Ojopi, E. B., Morya, E., Sameshima, K., & Ribeiro, S. (2015). Experience-dependent upregulation of multiple plasticity factors in the hippocampus during early REM sleep. Neurobiol Learn Mem, 122, 19-27. https://doi.org/10.1016/j.nlm.2015.01.002
Cerf-Ducastel, B., Van, D., Macleod, P., Le, B., & Faurion, A. (2001). Interaction of gustatory and lingual somatosensory perceptions at the cortical level in the human: a functional magnetic resonance imaging study. Chemical Senses., 26(4), 371-383. (NOT IN FILE)
Datta, S. (2000). Avoidance task training potentiates phasic pontine-wave density in the rat: A mechanism for sleep-dependent plasticity. Journal of Neuroscience, 20(22), 8607-8613. <Go to ISI>://000165131500047
Datta, S. (2010). Cellular and chemical neuroscience of mammalian sleep. Sleep Med, 11(5), 431-440. https://doi.org/10.1016/j.sleep.2010.02.002
Delacour, J. (1999). The memory system and brain organization: From animal to human studies. <Go to ISI>://WOS:000084508000012
Diekelmann, S., & Born, J. (2010). The memory function of sleep. Nat Rev Neurosci, 11(2), 114-126. https://doi.org/10.1038/nrn2762
Dudai, Y., Karni, A., & Born, J. (2015). The Consolidation and Transformation of Memory. Neuron, 88(1), 20-32. https://doi.org/10.1016/j.neuron.2015.09.004
Ecker, A. S., Berens, P., Keliris, G. A., Bethge, M., Logothetis, N. K., & Tolias, A. S. (2010). Decorrelated Neuronal Firing in Cortical Microcircuits [Report]. Science Magazine, 327(5965), 584-587. https://doi.org/10.1126/science.1179867
Ego-Stengel, V., & Wilson, M. A. (2010). Disruption of ripple-associated hippocampal activity during rest impairs spatial learning in the rat [Research Support, N.I.H., Extramural
Research Support, Non-U.S. Gov't]. Hippocampus, 20(1), 1-10. https://doi.org/10.1002/hipo.20707
Eschenko, O., Magri, C., Panzeri, S., & Sara, S. J. (2011). Noradrenergic Neurons of the Locus Coeruleus Are Phase Locked to Cortical Up-Down States during Sleep. Cereb Cortex. https://doi.org/10.1093/cercor/bhr121
Eschenko, O., Ramadan, W., Molle, M., Born, J., & Sara, S. J. (2008). Sustained increase in hippocampal sharp-wave ripple activity during slow-wave sleep after learning. Learning & Memory, 15(4), 222-228. https://doi.org/10.1101/lm.726008
Freeman, W. J. (2008). Making Sense of Brain Waves: The Most Baffling Frontier in Neuroscience. In P. Parelus, J. Principe, & S. Rajasekaran (Eds.), Biocomputing (pp. 33-55). Kluver.
Friston, K. J. (2002). Bayesian estimation of dynamical systems: an application to fMRI. Neuroimage., 16(2), 513-530. (NOT IN FILE)
Ghazanfar, A. A., & Logothetis, N. K. (2003). Facial expressions linked to monkey calls [Brief Communication]. Nature, 423(6943), 937-938. https://doi.org/10.1038/423937a
Girardeau, G., Benchenane, K., Wiener, S. I., Buzsaki, G., & Zugaro, M. B. (2009). Selective suppression of hippocampal ripples impairs spatial memory [Research Support, N.I.H., Extramural
Research Support, Non-U.S. Gov't]. Nat Neurosci, 12(10), 1222-1223. https://doi.org/10.1038/nn.2384
Goense, J., & Logothetis, N. K. (2008, May 3-9, 2008). Positive and negative BOLD-signals from blood vessels in monkey visual cortex ISMRM 16h Scientific Meeting and Exhibition, Toronto, ON, Canada.
Goense, J., Merkle, H., & Logothetis, N. K. (2012). High-Resolution fMRI Reveals Laminar Differences in Neurovascular Coupling between Positive and Negative BOLD Responses. Neuron, 76(3), 629-639. https://doi.org/DOI 10.1016/j.neuron.2012.09.019
Grosmark, A. D., Mizuseki, K., Pastalkova, E., Diba, K., & Buzsaki, G. (2012). REM sleep reorganizes hippocampal excitability. Neuron, 75(6), 1001-1007. https://doi.org/10.1016/j.neuron.2012.08.015
Gross, C. G., Bender, D. B., & Rocha-Miranda, C. E. (1969). Visual Receptive Fields of Neurons in Inferotemporal Cortex of the Monkey. Science, 166, 1303-1306. (NOT IN FILE)
Gross, C. G., Rocha-Miranda, C. E., & Bender, D. B. (1972). Visual properties of neurons in inferotemporal cortex of the Macaque. J Neurophysiol, 35(1), 96-111. https://doi.org/10.1152/jn.1972.35.1.96
Hindriks, R., Arsiwalla, X. D., Panagiotaropoulos, T., Besserve, M., Verschure, P. F., Logothetis, N. K., & Deco, G. (2016). Discrepancies between Multi-Electrode LFP and CSD Phase-Patterns: A Forward Modeling Study. Front Neural Circuits, 10, 51. https://doi.org/10.3389/fncir.2016.00051
Hoffman, K. L., & Logothetis, N. K. (2009). Cortical mechanisms of sensory learning and object recognition. Philos Trans R Soc Lond B Biol Sci, 364(1515), 321-329. https://doi.org/10.1098/rstb.2008.0271
Huerta, P. T., & Lisman, J. E. (1995). Bidirectional synaptic plasticity induced by a single burst during cholinergic theta oscillation in CA1 in vitro. Neuron, 15(5), 1053-1063. https://doi.org/10.1016/0896-6273(95)90094-2
Isomura, Y., Sirota, A., Ozen, S., Montgomery, S., Mizuseki, K., Henze, D. A., & Buzsaki, G. (2006). Integration and segregation of activity in entorhinal-hippocampal subregions by neocortical slow oscillations. Neuron, 52(5), 871-882. <Go to ISI>://000242856400014
Kann, O. (2011). The energy demand of fast neuronal network oscillations: insights from brain slice preparations. Frontiers in pharmacology, 2.
Kapoor, V., Dwarakanath, A., Safavi, S., Werner, J., Besserve, M., Panagiotaropoulos, T. I., & Logothetis, N. K. (2022). Decoding internally generated transitions of conscious contents in the prefrontal cortex without subjective reports. Nat Commun, 13(1), 1535. https://doi.org/10.1038/s41467-022-28897-2
Kayser, C., Petkov, C. I., Remedios, R., & Logothetis, N. K. (2011). Multisensory influences on auditory processing: Perspectives from fMRI and electrophysiology. In M. M. Murray & M. T. Wallace (Eds.), The Neural Bases of Multisensory Processes (pp. (in press)). CRC Press. http://www.crcpress.com/product/isbn/9781439812174?refpage=http%3A//www.crcpress.com/ecommerce_product/browse_book_categories.jsf&refpn=category&refpv=BIO01P
Leopold, D. A., & Logothetis, N. K. (1999). Multistable phenomena: Changing views in perception [Review]. Trends in Cognitive Sciences, 3(7), 254-264. https://doi.org/10.1016/S1364-6613(99)01332-7
Lippert, M. T., Steudel, T., Ohl, F., Logothetis, N. K., & Kayser, C. (2010). Coupling of neural activity and fMRI-BOLD in the motion area MT [Research Article]. Magnetic Resonance Imaging, 28(8), 1087-1094. https://doi.org/10.1016/j.mri.2009.12.028
Logothetis, N. K. (1998). Single units and conscious vision [Article]. Philosophical Transactions of the Royal Society London Series B - Biological Sciences, 353(1377), 1801-1818. <Go to ISI>://000077089300002
Logothetis, N. K. (2000). Object recognition: holistic representations in the monkey brain. Spat Vis, 13(2-3), 165-178. https://doi.org/10.1163/156856800741180
Logothetis, N. K. (2008). What we can do and what we cannot do with fMRI [Review]. Nature, 453(7197), 869-878. https://doi.org/10.1038/nature06976
Logothetis, N. K. (2015). Neural-Event-Triggered fMRI of large-scale neural networks. Curr Opin Neurobiol, 31, 214-222. https://doi.org/10.1016/j.conb.2014.11.009
Logothetis, N. K., Eschenko, O., Murayama, Y., Augath, M., Steudel, T., Evrard, H. C., Besserve, M., & Oeltermann, A. (2012). Hippocampal-cortical interaction during periods of subcortical silence. Nature, 491(7425), 547-553. https://doi.org/Doi 10.1038/Nature11618
Logothetis, N. K., & Pauls, J. (1995). Psychophysical and physiological evidence for viewer-centered object representations in the primate. Cereb Cortex, 5(3), 270-288. https://doi.org/10.1093/cercor/5.3.270
Logothetis, N. K., Pauls, J., Bulthoff, H. H., & Poggio, T. (1994). View-dependent object recognition by monkeys. Curr Biol, 4(5), 401-414. https://doi.org/10.1016/s0960-9822(00)00089-0
Logothetis, N. K., Pauls, J., & Poggio, T. (1995). Shape representation in the inferior temporal cortex of monkeys. Curr Biol, 5(5), 552-563. https://doi.org/10.1016/s0960-9822(95)00108-4
Logothetis, N. K., Pauls, J. M., Augath, M. A., Trinath, T., & Oeltermann, A. (2001). Neurophysiological investigation of the basis of the fMRI signal [Article]. Nature, 412(6843), 150-157. https://doi.org/10.1038/35084005
Logothetis, N. K., & Schall, J. D. (1989). Neuronal Correlates of Subjective Visual-Perception [Research Article]. Science, 245(4919), 761-763. https://doi.org/10.1126/science.2772635
Logothetis, N. K., & Sheinberg, D. L. (1996a). Visual object recognition. Annu Rev Neurosci, 19, 577-621. https://doi.org/10.1146/annurev.ne.19.030196.003045
Logothetis, N. K., & Sheinberg, D. L. (1996b). Visual object recognition. Annual Review of Neuroscience, 19, 577-621. https://doi.org/10.1146/annurev.ne.19.030196.003045
Lord, L. D., Expert, P., Huckins, J. F., & Turkheimer, F. E. (2013). Cerebral energy metabolism and the brain's functional network architecture: an integrative review. Journal of Cerebral Blood Flow and Metabolism, 33(9), 1347-1354. https://doi.org/DOI 10.1038/jcbfm.2013.94
Ludtke, N., Logothetis, N. K., & Panzeri, S. (2010). Testing methodologies for the nonlinear analysis of causal relationships in neurovascular coupling [Research article]. Magnetic Resonance Imaging, 28(8), 1113-1119. https://doi.org/10.1016/j.mri.2010.03.028
Magri, C., Whittingstall, K., Singh, V., Logothetis, N. K., & Panzeri, S. (2009, Sep 6-8, 2009). Information breakdown analysis of simultaneous neural recordings: tools for the study of neural codes 2nd INCF Congress of Neuroinformatics, Pilsen, Czech Republic.
Mazzoni, A., Panzeri, S., Logothetis, N. K., & Brunel, N. (2008). Encoding of Naturalistic Stimuli by Local Field Potential Spectra in Networks of Excitatory and Inhibitory Neurons [Research Article]. Plos Computational Biology, 4(12), e1000239. https://doi.org/10.1371/journal.pcbi.1000239
Mazzoni, A., Whittingstall, K., Brunel, N., Logothetis, N. K., & Panzeri, S. (2010). Understanding the relationships between spike rate and delta/gamma frequency bands of LFPs and EEGs using a local cortical network model [Article]. Neuroimage, 52(3), 956-972. https://doi.org/10.1016/j.neuroimage.2009.12.040
McCormick, D. A., & Bal, T. (1997). Sleep and arousal - Thalamocortical mechanisms. Annual Review of Neuroscience, 20, 185-215.
Miyauchi, S., Misaki, M., Kan, S., Fukunaga, T., & Koike, T. (2009). Human brain activity time-locked to rapid eye movements during REM sleep. Experimental Brain Research, 192(4), 657-667. https://doi.org/DOI 10.1007/s00221-008-1579-2
Molle, M., Marshall, L., Gais, S., & Born, J. (2002). Grouping of spindle activity during slow oscillations in human non-rapid eye movement sleep [Research Support, Non-U.S. Gov't]. J Neurosci, 22(24), 10941-10947. http://www.ncbi.nlm.nih.gov/pubmed/12486189
Molle, M., Yeshenko, O., Marshall, L., Sara, S. J., & Born, J. (2006). Hippocampal sharp wave-ripples linked to slow oscillations in rat slow-wave sleep. J Neurophysiol., 96(1), 62-70. http://www.ncbi.nlm.nih.gov/pubmed/16611848?dopt=Citation
O'Keefe, J., & Nadel, L. (1978). The Hippocampus as a Cognitive Map. Oxford University Press.
O'Neill, J., Senior, T. J., Allen, K., Huxter, J. R., & Csicsvari, J. (2008). Reactivation of experience-dependent cell assembly patterns in the hippocampus [Research Support, Non-U.S. Gov't]. Nat Neurosci, 11(2), 209-215. https://doi.org/10.1038/nn2037
Panagiotaropoulos, T. I., Kapoor, V., & Logothetis, N. K. (2014). Subjective visual perception: from local processing to emergent phenomena of brain activity. Philosophical Transactions of the Royal Society B-Biological Sciences, 369(1641), Article 20130534. https://doi.org/10.1098/rstb.2013.0534
Panagiotaropoulos, T. I., Kapoor, V., Logothetis, N. K., & Deco, G. (2013). A common neurodynamical mechanism could mediate externally induced and intrinsically generated transitions in visual awareness. Plos One, 8(1), e53833. https://doi.org/10.1371/journal.pone.0053833
PONE-D-12-32547 [pii]
Panzeri, S., Brunel, N., Logothetis, N. K., & Kayser, C. (2010). Sensory neural codes using multiplexed temporal scales [Review]. Trends in Neurosciences, 33(3), 111-120. https://doi.org/10.1016/j.tins.2009.12.001
Poe, G. R., Nitz, D. A., McNaughton, B. L., & Barnes, C. A. (2000). Experience-dependent phase-reversal of hippocampal neuron firing during REM sleep. Brain Res, 855(1), 176-180. https://doi.org/10.1016/s0006-8993(99)02310-0
Ramirez-Villegas, J. F., Besserve, M., Murayama, Y., Evrard, H. C., Oeltermann, A., & Logothetis, N. K. (2021). Coupling of hippocampal theta and ripples with pontogeniculooccipital waves. Nature, 589(7840), 96-102. https://doi.org/10.1038/s41586-020-2914-4
Rasch, B., Pommer, J., Diekelmann, S., & Born, J. (2009). Pharmacological REM sleep suppression paradoxically improves rather than impairs skill memory. Nat Neurosci, 12(4), 396-397. https://doi.org/10.1038/nn.2206
Ribeiro, S. (2012). Sleep and plasticity. Pflugers Archiv-European Journal of Physiology, 463(1), 111-120. https://doi.org/DOI 10.1007/s00424-011-1031-5
Ryali, S., Chen, T. W., Supekar, K., Tu, T., Kochalka, J., Cai, W. D., & Menon, V. (2016). Multivariate dynamical systems-based estimation of causal brain interactions in fMRI: Group-level validation using benchmark data, neurophysiological models and human connectome project data. Journal of Neuroscience Methods, 268, 142-153. https://doi.org/10.1016/j.jneumeth.2016.03.010
Safavi, S., Kapoor, V., Logothetis, N. K., & Panagiotaropoulos, T. I. (2014). Is the frontal lobe involved in conscious perception? Front Psychol, 5, 1063. https://doi.org/10.3389/fpsyg.2014.01063
Shmuel, A., Augath, M. A., Oeltermann, A., & Logothetis, N. K. (2006). Negative functional MRI response correlates with decreases in neuronal activity in monkey visual area V1 [Article]. Nature Neuroscience, 9(4), 569-577. https://doi.org/10.1038/nn1675
Siapas, A. G., & Wilson, M. A. (1998). Coordinated interactions between hippocampal ripples and cortical spindles during slow-wave sleep [Research Support, Non-U.S. Gov't
Research Support, U.S. Gov't, Non-P.H.S.]. Neuron, 21(5), 1123-1128. http://www.ncbi.nlm.nih.gov/pubmed/9856467
Sigala, N., & Logothetis, N. K. (2002). Visual categorization shapes feature selectivity in the primate temporal cortex. Nature, 415(6869), 318-320. https://doi.org/10.1038/415318a
Sirota, A., & Buzsáki, G. (2005). Interaction between neocortical and hippocampal networks via slow oscillations. Thalamus & related systems, 3(04), 245-259.
Sirota, A., Csicsvari, J., Buhl, D., & Buzsaki, G. (2003). Communication between neocortex and hippocampus during sleep in rodents. Proceedings of the National Academy of Sciences of the United States of America, 100(4), 2065-2069. https://doi.org/DOI 10.1073/pnas.0437938100
Soldatenko, S., & Yusupov, R. (2021). An Optimal Control Perspective on Weather and Climate Modification. Mathematics, 9(4). https://doi.org/ARTN 305
10.3390/math9040305
Steriade, M., & Llinas, R. R. (1988). The functional states of the thalamus and the associated neuronal interplay. [604 refs]. Physiological Reviews, 68(3), 649-742.
Steriade, M., Nunez, A., & Amzica, F. (1993). A novel slow (< 1 Hz) oscillation of neocortical neurons in vivo: depolarizing and hyperpolarizing components. Journal of Neuroscience, 13(8), 3252-3265.
Watanabe, M., Cheng, K., Murayama, Y., Ueno, K., Asamizuya, T., Tanaka, K., & Logothetis, N. (2011). Attention but not awareness modulates the BOLD signal in the human V1 during binocular suppression. Science, 334(6057), 829-831. https://doi.org/334/6057/829
Wierzynski, C. M., Lubenov, E. V., Gu, M., & Siapas, A. G. (2009). State-dependent spike-timing relationships between hippocampal and prefrontal circuits during sleep [Research Support, N.I.H., Extramural
Research Support, Non-U.S. Gov't]. Neuron, 61(4), 587-596. https://doi.org/10.1016/j.neuron.2009.01.011
Selected Articles
Neural Mechanisms of Bistable Perception
 Our research aim, both in Tübingen and Shanghai has been to study and understand what kind of neuronal activity changes underlie perceptual multistability, such as that experienced with the Necker cube shown on the left. We were and continue to be interested in this, as we believe that it is not just a quirk of our visual system. Instead, we are convinced that it tells us something about the organization of the entire brain and its way of making us aware of all sensory information. To make the perceptual task easy for the non-human primate, we decided to study the alternations experienced when two different visual patterns are presented simultaneously to each eye, a phenomenon called binocular rivalry (BR). Until the moment we started this research, the prevailing theory about BR was that it is a strictly a “binocular phenomenon” to optimize unified stereoscopic vision and utterly unrelated to other multistable perceptual phenomena.
Our research aim, both in Tübingen and Shanghai has been to study and understand what kind of neuronal activity changes underlie perceptual multistability, such as that experienced with the Necker cube shown on the left. We were and continue to be interested in this, as we believe that it is not just a quirk of our visual system. Instead, we are convinced that it tells us something about the organization of the entire brain and its way of making us aware of all sensory information. To make the perceptual task easy for the non-human primate, we decided to study the alternations experienced when two different visual patterns are presented simultaneously to each eye, a phenomenon called binocular rivalry (BR). Until the moment we started this research, the prevailing theory about BR was that it is a strictly a “binocular phenomenon” to optimize unified stereoscopic vision and utterly unrelated to other multistable perceptual phenomena.
Correspondingly, the site of perceptual suppression was thought to be in the primary visual cortex, instantiated in the strong mutual inhibition between orientation-selective cells, e.g. see representative review (Blake 1989). A few investigators, including Helmholtz, suggested that BR may be related to attention, but then many others used various psychophysical paradigms to further support the quasi-peripheral (i.e. primary rather than higher association cortices, or cortico-thalamo-cortical loops) origin of this phenomenon. In fact, the belief at that time was that information about the stimulus is entirely blocked after the input layers of V1, and thus is not available to other extrastriate areas such as V2, V4 or MT. Because neurons in the striate sublayers 4Ca and 4B are orientation- and direction-selective, and more than half of the cells in layers 4B and 4A are binocular, undiminished activity in layer 4 should be sufficient for generating the orientation and direction adaptation aftereffects, as well as their interocular transfer reported in a series of psychophysical studies.
To the best of our knowledge, our Science publication in 1989 was (a) the very first study correlating perception (rather than sensation) with physiology in monkeys and (b) the very first study that provided evidence that neuronal activity in the association visual cortex reflects the perceptual alternations reported by an animal experiencing binocular rivalry; importantly, with solid evidence that the monkey actually performs its task.
Neuronal Correlates of Subjective Visual Perception
Logothetis NK, Schall JD (1989) Science 245:761-763.
Experiments in the middle temporal area (MT or V5) revealed different cell populations whose activity was, to a greater or lesser extent, modulated in a complex way during binocular motion rivalry (Logothetis and Schall 1989). A number of cells appeared to fire only when the neuron’s preferred direction was perceived, others only when the stimulus was suppressed (Levelt’s second proposition). Other neurons were found to be active only during the perceptual (rather than physical) alternation of the stimulus. The study drew a great deal of attention both in the popular and the scientific press, including a first discussion by Benjamin Libet on the implications of the study for conscious perception; see response in (Logothetis 1990). Yet MT neurons receive direct input from both layers 4B and 6 of the striate cortex! It was thus still not clear whether perception-related response modulation in MT was a property of this area or reflected activity changes occurring in striate cortex.
Activity changes in early visual cortex reflect monkeys' percepts during binocular rivalry
Leopold DA, Logothetis NK (1996) Nature 379:549-553
To determine whether perception-related modulation of activity occurs in other visual cortical areas, we recorded from individual neurons in V1, V2 and V4 while monkeys reported the perceived orientation of rival gratings of two orthogonal orientations. Many cells, particularly in V4, showed patterns of activity that correlated with the perceptual dominance and suppression of one stimulus. The majority were orientation-selective and could be driven equally well from either eye. It has been previously suggested that binocular rivalry involves reciprocal inhibition between monocular neurons within V1, but our results do not support this view. Instead, we propose that binocular rivalry arises through interactions between binocular neurons at several levels in the visual pathways, and that similar mechanisms may underlie other multistable perceptual states that occur when viewing ambiguous images (Leopold and Logothetis 1996).
Following the publication of this paper, Francis Crick, in a commentary to Nature, stated that “although a casual reader might not realize it, these two papers (Logothetis and Schall 1989, Leopold and Logothetis 1996) are among the opening salvoes of a concerted attack on the baffling problem of consciousness. For this task the primate visual system seems especially attractive. The two papers show one direct approach to the study of neural correlates of consciousness .” His comments were very flattering, of course, but in all honesty, our work did instigate more commentaries and articles in that vein than I can possibly remember. This was encouraging indeed; though rest assured: “consciousness” still remains a mystery! About a year later, I published the first clear evidence that rivalry reflects the alternation of pattern rather than eye dominance (Logothetis, Leopold et al. 1996)
What is rivaling during binocular rivalry?
Logothetis NK, Leopold DA, Sheinberg DL (1996) Nature 380:621-624.
Together with David Sheinberg and David Leopold, at the time postdoc and student, respectively, I tested the effect of rapidly alternating the rival stimuli between the two eyes. Under these conditions, the perceptual alternations exhibit the same temporal dynamics as with static patterns, and a single phase of perceptual dominance can span multiple alternations of the stimuli. Thus, neural representations of the two stimuli compete for visual awareness independently of the eye through which they reach the higher visual areas. This finding placed binocular rivalry in the general category of multistable phenomena such as ambiguous figures, and it provided a new way to study the neural cause and resolution of perceptual ambiguities. At the same time, we had started recordings in the inferior temporal cortex of monkeys (Logothetis, Leopold et al. 1996).
The role of temporal cortical areas in perceptual organization
Sheinberg D, Logothetis NK (1997) PNAS, 94:3408-3413.
 The visual areas of the temporal lobe of the primate were always thought to be essential for the representation of visual objects. To examine the role of these areas in the visual awareness of a stimulus, we recorded the activity of single neurons in monkeys trained to precisely report their percepts when viewing ambiguous stimuli. As mentioned above, our first recordings in areas V1, V2, V4, and MT of monkeys experiencing binocular rivalry showed that only a small proportion of striate and early extrastriate neurons discharge exclusively when the driving stimulus is seen. In contrast, the activity of almost all neurons in the inferior temporal cortex and the visual areas of the cortex of superior temporal sulcus was found to be contingent upon the perceptual dominance of an effective visual stimulus. These areas thus appear to represent a stage of processing beyond the resolution of ambiguities, and thus beyond the processes of perceptual grouping and image segmentation, where neural activity reflects the brain's internal view of objects rather than the effects of the retinal stimulus on cells encoding simple visual features or shape primitives.
The visual areas of the temporal lobe of the primate were always thought to be essential for the representation of visual objects. To examine the role of these areas in the visual awareness of a stimulus, we recorded the activity of single neurons in monkeys trained to precisely report their percepts when viewing ambiguous stimuli. As mentioned above, our first recordings in areas V1, V2, V4, and MT of monkeys experiencing binocular rivalry showed that only a small proportion of striate and early extrastriate neurons discharge exclusively when the driving stimulus is seen. In contrast, the activity of almost all neurons in the inferior temporal cortex and the visual areas of the cortex of superior temporal sulcus was found to be contingent upon the perceptual dominance of an effective visual stimulus. These areas thus appear to represent a stage of processing beyond the resolution of ambiguities, and thus beyond the processes of perceptual grouping and image segmentation, where neural activity reflects the brain's internal view of objects rather than the effects of the retinal stimulus on cells encoding simple visual features or shape primitives.
Interestingly, there are conditions under which perceptual alternation can be slowed down or even stopped. For instance, when the dichoptic patterns are viewed intermittently, either by repetitive presentation or by periodic closing of the eyes, perception can become locked or "frozen" in one configuration for several minutes at a time (Leopold, Wilke et al. 2002, Maier, Wilke et al. 2003). One aspect of this stabilization is the possible existence of a perceptual memory that persists during periods in which the ambiguous stimulus is absent. Such memory may actually be important in natural vision. We further propose that the interleaved paradigm introduced here may be of great value in gauging aspects of stimulus similarity that appeal to particular mechanisms of perceptual organization.
All in all, I believe our studies on perceptual ambiguity were able to shed some new light on the phenomenon of binocular rivalry and visual perception in general. They triggered a great deal of research in psychophysics, physiology and cognitive sciences using diverse methods such as EEG, MEG and fMRI. I mention here in passing that in the period from 1921 to 1988 there were only 202 publications on binocular rivalry, the vast majority of which concentrated on the role of the phenomenon in cyclopean vision. In the time period that followed our paper, namely in the years 1989 to 2011, the number of publications exceeded 1200, with our work being cited about 2000 times. Ever since, the discussion has been concentrated on the central origin of perceptual alternations and the capacity of the phenomenon to offer insights into conscious perception.
 As a matter of fact, it is now generally accepted (even by those who initially strongly advocated inter-ocular rather than inter-precept competition, e.g. R. Blake, H. Wilson, and others) that rivalry does not really reflect interocular competition. Interocular inhibitory interactions most likely contribute to the local conflicts that arise when two incompatible stimuli are superimposed. Such conflicts may arise with or without dichoptic stimulation and are most likely the origin of the instability experienced during binocular or monocular rivalry. However, it is very unlikely that they can explain stimulus selection and percept generation. The dominance and suppression of a pattern during rivalry reflects the excitation and inhibition of cell populations in the higher visual areas, which are directly involved in the representation of visual patterns. At the moment, we are conducting a large number of concurrent psychophysics, physiology and fMRI studies in humans and monkeys in order to better understand the topology of the networks involved in the perceptual dominance and suppression of a stimulus, and will then study the behavior of cortical microcircuits during the alternation of perception.
As a matter of fact, it is now generally accepted (even by those who initially strongly advocated inter-ocular rather than inter-precept competition, e.g. R. Blake, H. Wilson, and others) that rivalry does not really reflect interocular competition. Interocular inhibitory interactions most likely contribute to the local conflicts that arise when two incompatible stimuli are superimposed. Such conflicts may arise with or without dichoptic stimulation and are most likely the origin of the instability experienced during binocular or monocular rivalry. However, it is very unlikely that they can explain stimulus selection and percept generation. The dominance and suppression of a pattern during rivalry reflects the excitation and inhibition of cell populations in the higher visual areas, which are directly involved in the representation of visual patterns. At the moment, we are conducting a large number of concurrent psychophysics, physiology and fMRI studies in humans and monkeys in order to better understand the topology of the networks involved in the perceptual dominance and suppression of a stimulus, and will then study the behavior of cortical microcircuits during the alternation of perception.
Neural Mechanisms Underlying Object recognition
From the beginning, bistable perception has been one of my two main research interests. The second is visual object representation, memory, and recognition. Many of the ideas and experiments have been described in some detail in previous reviews (Logothetis and Sheinberg 1996, Logothetis 1998, Gauthier and Logothetis 2000, Logothetis 2000). Here I will refer selectively to some landmark and most-cited papers on the physiology of the inferior temporal cortex.
Visual Object Recognition
Logothetis NK and Sheinberg, DL. (996) Annual Review of Neuroscience 10: 577-621
In this review, we considered evidence from the fields of psychology, neuropsychology, and neurophysiology, all of which supported the idea that there are multiple systems for recognition. Data from normal adults, infants, animals, and brain-damaged patients reveal a major distinction between the classification of objects at a basic category level and the identification of individual objects from a homogeneous object class. An additional distinction between object representations used for visual perception and those used for visually guided movements provided further evidence of a multiplicity of visual recognition systems. Moreover, our psychophysical and neurophysiological studies indicated that one system may represent objects by combinations of multiple views, or aspects, while another may represent objects by structural primitives and their spatial interrelationships.
We started our research on recognition by first examining in great detail the performance of macaques in categorization and identification tasks. Following extensive training, the animals learned to discriminate individual objects from a set of highly similar distracters, a task not unlike the problem of identifying a specific face or a particular bird species (so-called “expert behavior”). Their ability to generalize from known to novel views was found to be almost identical to that of expert humans. Subsequent physiological recordings from individual neurons in the inferior temporal lobe, near the anterior medial temporal sulcus (AMTS), yielded some interesting results (Logothetis and Sheinberg 1996)
Shape Representation in the Inferior Temporal Cortex of Monkeys
Logothetis NK, Pauls JM, Poggio T (1995) Current Biol 5:552-563
We found a population of IT neurons that responded selectively to views of previously unfamiliar objects. The cells discharged maximally to one view of an object, and their response declined gradually as the object was rotated away from this preferred view. No selective responses were ever encountered-for views that the animal systematically failed to recognize. Most neurons also exhibited orientation-dependent responses during view-plane rotations. Some neurons were found to be tuned around two views of the same object, and a very small number of cells responded in a view-invariant manner. For the five different objects that were used extensively during the training of the animals, and for which behavioral performance became view-independent, multiple cells were found that were tuned around different views of the same object. A number of view-selective units showed response invariance for changes in the size of the object or the position of its image within the parafovea.
These results suggested that IT neurons can actually develop a complex receptive field organization as a consequence of extensive training in the discrimination and recognition of objects. None of these objects had any prior meaning for the animal, nor did they resemble anything familiar in the monkey's environment. Simple geometric features did not appear to account for the neurons' selective responses. These findings strongly supported the idea that a population of neurons - each tuned to a different object aspect and each showing a certain degree of invariance to image transformations - may, as an ensemble, encode at least some types of complex three-dimensional objects. In such a system, several neurons may be active for any given vantage point, with a single unit acting like a blurred template for a limited neighborhood of a single view (Logothetis, Pauls et al. 1994, Logothetis and Pauls 1995, Logothetis, Pauls et al. 1995).
In a continuation of this line of investigation, we tested how different theoretical models of object recognition account for expert behavior in both humans and monkeys and what happens in the monkey brain during the process of training and familiarization (Sigala, Gabbiani et al. 2002, Sigala and Logothetis 2002). The results indicated – once again – important similarities between human and monkey recognition strategies. Neither species compared the new stimuli to class prototypes or based their decisions on conditional probabilities along stimulus dimensions. Instead, they classified each object according to its similarity to familiar members of the alternative categories, or with respect to its proximity to a linear boundary between the learned categories.
Visual categorization shapes feature selectivity in the primate temporal cortex
Sigala N, Logothetis NK (2002) Nature 415 :318-320
The first series of experiments on categorization relied on processing of holistic information. We also conducted recognition experiments in monkeys trained to categorize parameterized stimuli, e.g. line drawings of faces or fish. Each schematic stimulus consisted of an outline and four varying features, e.g. eye height, eye separation, nose length and mouth height for the face drawings. Features could take three discrete values and the categories were separable along two of the four dimensions of the stimuli. In the face drawings, for instance, diagnostic dimensions were eye height and separation. Electrophysiological recordings showed that neurons in the ITC did indeed react selectively to diagnostic features (Sigala and Logothetis 2002). Obviously, such a priori parameterization cannot be done easily when the stimuli are complex objects or entire scenes. Natural images contain structure on many spatial scales distributed nonhomogenously across the image and are thus good examples of complex, redundant visual forms. We therefore further identified diagnostic fragments by presenting stimuli behind occluders, i.e. mid-gray masks punctured by a number of randomly located windows (‘‘bubbles’’) through which the occluded image is visible. The monkeys continued to perform their discrimination task on the partially visible images. Whether they could identify the partially visible stimuli depended on whether the occluder uncovered image parts critical for task performance. Diagnostic feature selectivity was found in both the MUA and the LFP responses, suggesting again that such features are explicitly encoded in the response of neurons (Nielsen, Logothetis et al. 2006, Nielsen, Logothetis et al. 2006, Nielsen, Logothetis et al. 2008). However, while MUA was homogenously distributed across the tested portion of ITC, LFP were selective only in the anteriorly located recording sites, a finding suggesting that diagnosticity is first encoded in the posterior IT cortex, and demonstrating the power of combined analysis of field potentials and spiking activity for mapping structure to computational function in the brain.
Three-dimensional shape representation in monkey cortex
Sereno, M. E., Trinath, T., Augath, M. A., & Logothetis, N. K. (2002). Neuron, 33(4), 635-652
 The representation of shape and objects was also studied in fMRI experiments with anesthetized or behaving monkeys. In one series of experiments, computer-generated 3-D objects defined by shading, random dots, structure elements or silhouettes were presented either statically or dynamically (rotating). Our findings suggest that 3-D shape representations are highly localized although widely distributed in occipital, temporal, parietal and frontal cortices, and may involve common brain regions regardless of shape cue (Sereno, Trinath et al. 2002). Ongoing work examines the specificity of this network across different experimental subjects.
The representation of shape and objects was also studied in fMRI experiments with anesthetized or behaving monkeys. In one series of experiments, computer-generated 3-D objects defined by shading, random dots, structure elements or silhouettes were presented either statically or dynamically (rotating). Our findings suggest that 3-D shape representations are highly localized although widely distributed in occipital, temporal, parietal and frontal cortices, and may involve common brain regions regardless of shape cue (Sereno, Trinath et al. 2002). Ongoing work examines the specificity of this network across different experimental subjects.
Neurovascular Coupling
Brains can be thought of as complex adaptive systems both in the intuitive sense and in scientific-computational terms. They have very high structural complexity and massive connectivity, both of which change and evolve in response to experience. Information related to sensors and effectors is processed in both a parallel and a hierarchical fashion. The connectivity between different hierarchical levels is bidirectional, and its effectiveness is continuously controlled by specific associational and neuromodulatory centers.
One central difficulty in studying such systems is adequately defining an elementary operational unit, as any such module can be a complex system and may be recursively decomposed into other sets of units. When questions are addressed at the level of a distributed large-scale system such as that underlying perception and cognition (i.e. the capacities I am interested in studying), single neurons – often thought of as the “obvious” functional units of the brain – can hardly be informative modules.
Localizing and comprehending the neural mechanism underlying our perceptual or cognitive capacities is likely to require knowledge both of synergistic population activity and of local and long-range interconnectivity between modules. In fact, in complex systems the relationships between the elementary modules may be more important than the modules themselves.
I firmly believe that a multimodal approach has become utterly indispensable for the study of the brain’s function and dysfunction. In this context, it can be very helpful to combine global imaging technologies such as MRI, PET and MEG with invasive methods affording us direct access to the brain’s electrical activity.
We need to understand the organization of microcircuits, but we also need a profound understanding of their role in widespread networks. Input, state and context-dependent effective connectivity is essential for the emergence of behavior-related patterns and of disseminated feedback. It can also be critical for the planning and design of multiple-site and multiple-electrode physiological recordings.
The above brief description explains the motivation behind my efforts over the last 15 years or so to develop a system that permits simultaneous measurements of MR imaging and electrophysiology. Unfortunately, however, MRI can only measure surrogate signals such as blood flow, volume and oxygenation; the exact relationship between such metabolic/hemodynamic parameters and the underlying neural responses remains largely elusive. I therefore quickly found myself deviating somewhat from my main aim, and asking elementary questions related to the mechanisms of neurovascular coupling (NVC). Naturally, before trying to interpret any kind of activation map, I felt I needed to know what causes “blobs” of activation.
To address a number of elementary questions related to NVC we have modified and further developed three complete scanner systems that currently permit simultaneous fMRI and electrophysiology, neuropharmacology, mass spectroscopy-neurochemistry, and electrical stimulation. For experiment with behaving animals we have also developed all the hardware and software required to measure eye, jaw, and body movements and to use these sensor signals to shape and train the behavior of the animals.
In order to combine electrophysiology and fMRI we developed the entire interference-compensation system and implemented it in both the 4.7T and the two 7T magnets. This system permits the uninterrupted recording of neural activity by measuring and compensating all current induced by the alternating magnet gradients.
Currently, up to 15 channels can be used to recording signals from different brain sites distributed in a tangential or radial direction. To allow fMRI of front-temporal areas, we also had to optimize pulse sequences and to develop a number of different types of regular or implantable radiofrequency coils which enable resolution on the scale of 0.1x0.1x0.6 mm.
All in all, when we started our “multimodal approach”, we bought “magnets” and their console. Every single additional hardware was designed and built in the lab. All of these technological achievements have been described in numerous publications in technical journals and occasionally even in high-impact periodicals like Neuron. In the following I summarize a small selection of landmark papers which, in my opinion, made a certain contribution to our understanding of NVC.
Functional imaging of the monkey brain
Logothetis NK, Guggenberger H, Peled S, Pauls J (1999) Nature Neuroscience 2: 555-62
 This was the first fMRI study in anesthetized and alert monkeys (Logothetis, Guggenberger et al. 1999). We presented spatially highly resolved functional images of the monkey cortex based on blood oxygenation level dependent (BOLD) contrast.
This was the first fMRI study in anesthetized and alert monkeys (Logothetis, Guggenberger et al. 1999). We presented spatially highly resolved functional images of the monkey cortex based on blood oxygenation level dependent (BOLD) contrast.
Checkerboard patterns or pictures of primates were used to study stimulus-induced activation of the visual cortex in a 4.7-Tesla magnetic field; we used optimized multi-slice, gradient-recalled, echo-planar imaging (EPI) sequences to image the entire brain. Under our anesthesia protocol, visual stimulation yielded robust, reproducible, focal activation of the lateral geniculate nucleus (LGN), the primary visual area (V1) and a number of extrastriate visual areas, including areas in the superior temporal sulcus. Similar responses were obtained in alert, behaving monkeys performing a discrimination task.
Several commentaries, were written about this study, e.g. Paradiso MA (1999) Monkey business builds a bridge to the human brain. Nature Neuroscience 2: 491-492.
Neurophysiological investigation of the basis of the fMRI signal
Logothetis NK, Pauls JM, Augath MA, Trinath T, Oeltermann A (2001) Nature 412:150-157
This study presents the first concurrent intracortical recordings of neural signals and fMRI responses (Logothetis, Pauls et al. 2001). We compared local field potentials (LFPs) as well as single- and multi-unit spiking activity with highly spatio-temporally resolved blood-oxygen-level-dependent (BOLD) fMRI responses from the visual cortex of monkeys. The largest magnitude changes were observed in LFPs, which at recording sites characterized by transient responses were the only signal that significantly correlated with the hemodynamic response. Linear systems analysis on a trial-by-trial basis showed that the impulse response of the neurovascular system is both animal- and site-specific, and that LFPs yield a better estimate of BOLD responses than the multi-unit responses. These findings suggest that the BOLD contrast mechanism reflects the input and intracortical processing of a given area rather than its spiking output. There have been countless commentaries and report about this study, including those of Raichle ME (2001); Charvy Narain (2006) Nature Neuroscience; and Bandettini PA, Ungerleider LG (2001), and public press. To date the study has been cited about 1,800 times.
Negative functional MRI response correlates with decreases in neuronal activity in monkey visual area V1
Shmuel A, Augath MA, Oeltermann A, Logothetis NK (2006) Nat Neuro 9:569-577
This is the first study on the neural mechanisms of the sustained negative BOLD responses (deactivations). Through simultaneous functional magnetic resonance imaging and electrophysiological recording, we demonstrated a negative BOLD response (NBR) beyond the stimulated regions of visual cortex that is associated with local decreases in neuronal activity below spontaneous activity and detected 7.15 +/- 3.14 mm away from the closest positively responding region in V1. Trial-by-trial amplitude fluctuations revealed tight coupling between the NBR and neuronal activity decreases. The NBR was associated with comparable decreases in local field potentials and multiunit activity. Our findings indicate that a significant component of the NBR originates in neuronal activity decreases
Neurophysiology of the BOLD fMRI Signal in Awake Monkeys
Goense JBM, Logothetis NK (2008) Current Biol 18:631-640
One important question raised by the aforementioned investigations is whether the reported correlations between neural and hemodynamic signals also apply to alert subjects. In this study, we trained monkeys to perform a fixation task while stimuli within the receptive field of each recording site were used to elicit neural responses followed by a BOLD response. We showed – for the first time in alert behaving monkeys as well - that although both LFP and MUA make significant contributions to the BOLD response, LFPs are better and more reliable predictors of the BOLD signal. Moreover, when MUA responses adapt but LFP remains unaffected, the BOLD signal remains unaltered. BOLD, if it is indeed primarily determined by the local processing of inputs in a given cortical area, does not reflect the activity of the usual stimulus- or task-related neurons reported in electrophysiological experiments (Goense and Logothetis 2008).
The effect of a serotonin-induced dissociation between spiking and perisynaptic activity on BOLD functional MRI
Rauch A, Rainer G, Logothetis NK (2008) PNAS 105:6759-6764
This was the first study using simultaneous recording of BOLD and electrophysiological activity while inducing a dissociation of MUA from LFP activity with injections of the neuromodulator BP554 into the primary visual cortex of anesthetized monkeys. BP554 is a 5-HT1A agonist acting primarily on the membrane of efferent neurons by potassium-induced hyperpolarization. Its infusion in visual cortex reliably reduced MUA without affecting either UP or BOLD activity. This finding suggested that the efferents of a neuronal network produce relatively little metabolic burden compared with the overall presynaptic and postsynaptic processing of incoming afferents.
The microvascular system of the striate and extrastriate visual cortex of the macaque
Weber, B., Keller, A. L., Reichold, J., & Logothetis, N. K. (2008) Cerebral Cortex, 18(10), 2318-2330
The aim of this study was to characterize the microvascular system of the primate cortex as a basis for understanding the constraints imposed on a region's hemodynamic response by its vascular architecture, density, as well as area- and layer-specific variations. In the macaque visual cortex, an array of anatomical techniques was applied, including corrosion casts, immunohistochemistry, and cytochrome oxidase (COX) staining. Detailed measurements of regional vascular length density, volume fraction, and surface density revealed a similar vascularization in different visual areas. Whereas the lower cortical layers showed a positive correlation between vascular and cell density, this relationship was very weak in the upper layers. Synapse density values taken from the literature also displayed a very moderate correlation with the vascular density. However,  vascular density was strongly correlated with the steady-state metabolic demand as measured by COX activity. This observation suggests that although the number of neurons and synapses determines an upper bound on an area's integrative capacity, its vascularization reflects the neural activity of those subpopulations that represent a "default" mode of brain steady state (Weber, Keller et al. 2008, Keller, Schüz et al. 2011)
vascular density was strongly correlated with the steady-state metabolic demand as measured by COX activity. This observation suggests that although the number of neurons and synapses determines an upper bound on an area's integrative capacity, its vascularization reflects the neural activity of those subpopulations that represent a "default" mode of brain steady state (Weber, Keller et al. 2008, Keller, Schüz et al. 2011)
In vivo Connectivity
As mentioned above, in vivo connectivity can provide valuable information regarding the topology and functional activity of large-scale networks that are activated during the execution of a cognitive task. To study such connectivity, one can use methods like diffusion tensor imaging (DTI), manganese enhanced MRI (MEMRI) and direct electrical stimulation and fMRI (DES-fMRI). All these techniques are non-destructive and can be used in longitudinal experiments on plasticity, memory formation, and learning. DTI exploits the diffusion anisotropy of water, while MEMRI uses the transneuronal transfer of manganese. In our lab, we have been using this technique for almost 10 years, and our recent employment of osmotic pumps and optimized injection protocols now permit its repeated use in rats and monkeys with no tissue damage. We are currently using MEMRI to trace neuromodulatory and cortico-subcortico-cortical pathways.
In vivo connectivity can be also studied with DES-fMRI. This technique permits the investigation of many different large-scale interactions, including the organization of projective fields, large-scale effects of local synaptic plasticity changes, and spatio-temporal profile of neuromodulatory effects induced by diffuse ascending systems. The methodology is sure to have translational value, too, as it allows the study of the function and dysfunction of networks subserving cognition and can provide insights into the mechanisms of electrotherapy and assist the design of neural prosthetic systems. Over the last 6 years, we have developed and optimized DES-fMRI for the anesthetized and behaving animal and used it to map activity during cortical stimulation and to study ES-induced signal propagation as well as the effects of LTP on hippocampus-cortical connectivity.
Mapping cortical activity elicited with electrical microstimulation using fMRI in the macaque
Tolias, A. S., Sultan, F. R., Augath, M. A., Oeltermann, A., Tehovnik, E. J., Schiller, P. H., & Logothetis, N. K. (2005) Neuron, 48(6)
In this study we first demonstrated the feasibility of the method, and subsequently we showed that the spatial extent of microstimulation exceeds what might be expected from passive spread of the current, suggesting a synaptic propagation of activity via horizontal connections. During V1 stimulation, a positive BOLD response was also observed in areas V2, V3, V4, and MT/V5, all of which are monosynaptically connected to V1. Because these extrastriate areas are reciprocally connected to V1, it is of course impossible to determine from the fMRI whether the observed extrastriate activity was driven orthodromically via V1’s input to these regions, or by direct antidromic activation of their projections to V1. Most importantly, however, the first study failed to reveal multisynaptic activations (Tolias, Sultan et al. 2005).
The effects of electrical microstimulation on cortical signal propagation
Logothetis NK, Augath MA, Murayama Y, Rauch A, Sultan FR, Goense JBM, et al.(2010) Nat Neuro 13:1283-1291.
In order to further elucidate the aforementioned observation, we examined the cortical activity patterns elicited during the stimulation of cortical afferents in monkeys. We found that stimulation of a site in the lateral geniculate nucleus (LGN) increased the fMRI signal in the regions of primary visual cortex (V1) that received input from that site, but suppressed it in the retinotopically matched regions of extrastriate cortex. Consistent with previous observations, intracranial recordings indicated that a short excitatory response occurring immediately after a stimulation pulse was followed by a long-lasting inhibition. Following microinjections of GABA antagonists in V1, LGN stimulation induced positive fMRI signals in all of the cortical areas. Taken together, our findings suggest that electrical stimulation disrupts cortico-cortical signal propagation by silencing the output of any neocortical area whose afferents are electrically stimulated. In their recent review on electrical stimulation, Clark, Armstrong and Moore (Clark, Armstrong et al. 2011) note that if this finding generalizes to all cortical and subcortical stimulation sites, it will have important implications for interpreting the results and inferring the likely neural substrate of microstimulation’ s behavioral effects in numerous previous experiments. Indeed, the BOLD maps generated during electrical stimulation may reveal areas that have lost afferent signals rather than projection fields and regions involved in certain types of cognitive behavior. Behaviors induced by microstimulation, whether directly by DES or indirectly via transcranial magnetic stimulation (TMS), may reflect processing in cortico-subcortico-cortical pathways rather than direct cortico-cortical communication.
A Small Selection of Methodological Advancements
The Influence of Moderate Hypercapnia on Neural Activity in the Anesthetized Nonhuman Primate
Zappe AC, Uludag K, Oeltermann A, Ugurbil K, Logothetis NK (2008) Cereb Cortex 18:2666-2673
In this study, we used combined intracortical recordings and fMRI in the visual cortex of anesthetized macaque monkeys to examine whether spontaneous neuronal activity is in fact significantly reduced by the moderate hypercapnia used to “calibrate” the BOLD signal. As expected, measurement of cerebral blood volume using an exogenous contrast agent and of BOLD signal showed that both are increased during hypercapnia. In contrast to this, spontaneous fluctuations of local field potentials in the beta and gamma frequency range as well as multiunit activity are reduced by approx. 15% during inhalation of 6% CO2. A strong tendency toward a reduction of neuronal activity was also found during inhalation of 3% CO2. These findings suggest that CMRO2 might be reduced during hypercapnia and caution must be exercised when hypercapnia is applied to calibrate the BOLD signal (Zappe, Uludag et al. 2008)
The effect of labeling parameters on perfusion-based fMRI in nonhuman primates
Zappe, A. C., Pfeuffer, J., Merkle, H., Logothetis, N. K., & Goense, J. B. M. (2008) CBFM, 28(3), 640-652.
 The blood oxygenation level-dependent (BOLD) signal is the most commonly used modality of functional magnetic resonance imaging (fMRI) today. Although easy to implement, it is an ambiguous signal since it results from a combination of several hemodynamic factors. Functional cerebral blood flow changes, as measured using arterial spin labeling (ASL), typically occur in the parenchyma and have been demonstrated to be more closely coupled to neural activation than BOLD. However, the intrinsically low signals from ASL techniques have hindered its widespread application to fMRI for basic research and even more so for clinical applications. Here, we report the first implementation of continuous ASL in the anesthetized macaque at high magnetic field of 7 T. The technique was optimized to permit maximum signal-to-noise ratio of functional perfusion-based images at high spatial resolution. The effect of labeling parameters such as label time and post-label delay (PLD) on functional cerebral blood flow (fCBF) in the visual cortex was evaluated. Functional cerebral blood flow maps did not change with increasing label time after 2,000ms, indicating that a label time of 2,000 ms is sufficient for reliable mapping of fCBF. The percent changes obtained using fCBF were better localized to gray matter than those obtained with BOLD. A short PLD of 200 ms revealed significantly higher fCBF changes at the cortical surface, indicating large-vessel contamination, than a long PLD of 800 ms. However, the effect of the PLD on fCBF was smaller than on baseline CBF. These results are of importance for high-resolution applications and when accurate quantification is required for studies in monkeys or humans (Zappe, Reichold et al. 2007, Zappe, Pfeuffer et al. 2008).
The blood oxygenation level-dependent (BOLD) signal is the most commonly used modality of functional magnetic resonance imaging (fMRI) today. Although easy to implement, it is an ambiguous signal since it results from a combination of several hemodynamic factors. Functional cerebral blood flow changes, as measured using arterial spin labeling (ASL), typically occur in the parenchyma and have been demonstrated to be more closely coupled to neural activation than BOLD. However, the intrinsically low signals from ASL techniques have hindered its widespread application to fMRI for basic research and even more so for clinical applications. Here, we report the first implementation of continuous ASL in the anesthetized macaque at high magnetic field of 7 T. The technique was optimized to permit maximum signal-to-noise ratio of functional perfusion-based images at high spatial resolution. The effect of labeling parameters such as label time and post-label delay (PLD) on functional cerebral blood flow (fCBF) in the visual cortex was evaluated. Functional cerebral blood flow maps did not change with increasing label time after 2,000ms, indicating that a label time of 2,000 ms is sufficient for reliable mapping of fCBF. The percent changes obtained using fCBF were better localized to gray matter than those obtained with BOLD. A short PLD of 200 ms revealed significantly higher fCBF changes at the cortical surface, indicating large-vessel contamination, than a long PLD of 800 ms. However, the effect of the PLD on fCBF was smaller than on baseline CBF. These results are of importance for high-resolution applications and when accurate quantification is required for studies in monkeys or humans (Zappe, Reichold et al. 2007, Zappe, Pfeuffer et al. 2008).
fMRI of the temporal lobe of the awake monkey at 7T
Goense, J. B. M., Ku, S. P., Merkle, H., Tolias, A. S., & Logothetis, N. K. (2008). NeuroImage, 39(3), 1081-1093
 Increasingly, 7 T scanners are being used for fMRI of humans and non-human primates, promising improvements in signal-to-noise ratio, spatial resolution and specificity. One disadvantage of fMRI at 7T is that susceptibility artifacts from air-filled cavities like the ear canal and nasal cavity cause signal loss and distortion. This limits the applicability of fMRI in these areas, but it also limits the study of processes that span large-scale cortical networks or the entire brain. Our goal is to study the inferior temporal (IT) lobe in awake monkeys because of its importance in object perception and recognition, but the functional signal is degraded by susceptibility gradients. To allow fMRI of this region, we optimized SE-EPI, which recovers signals lost with GE-EPI, and we corrected image distortions. SE-EPI (but not GE-EPI) has the additional advantage that the functional signal arises from the microvasculature and hence better represents neural activation. We show fMRI at 7 T of the entire visual pathway in the awake primate with robust and widespread activation in all ventral areas of the brain, including areas adjacent to the ear canal. This allows fMRI of areas that normally suffer from artifacts and thus more reliable whole-brain studies (Goense, Ku et al. 2008, Ku, Tolias et al. 2011)
Increasingly, 7 T scanners are being used for fMRI of humans and non-human primates, promising improvements in signal-to-noise ratio, spatial resolution and specificity. One disadvantage of fMRI at 7T is that susceptibility artifacts from air-filled cavities like the ear canal and nasal cavity cause signal loss and distortion. This limits the applicability of fMRI in these areas, but it also limits the study of processes that span large-scale cortical networks or the entire brain. Our goal is to study the inferior temporal (IT) lobe in awake monkeys because of its importance in object perception and recognition, but the functional signal is degraded by susceptibility gradients. To allow fMRI of this region, we optimized SE-EPI, which recovers signals lost with GE-EPI, and we corrected image distortions. SE-EPI (but not GE-EPI) has the additional advantage that the functional signal arises from the microvasculature and hence better represents neural activation. We show fMRI at 7 T of the entire visual pathway in the awake primate with robust and widespread activation in all ventral areas of the brain, including areas adjacent to the ear canal. This allows fMRI of areas that normally suffer from artifacts and thus more reliable whole-brain studies (Goense, Ku et al. 2008, Ku, Tolias et al. 2011)
Interpretation of Neural Signals
Spiking activity captures the output of individual neurons. Local field potentials (LFPs), on the other hand, capture massed synaptic activity and other slow aspects of the activity of large local populations. Recording all these electrophysiological signals as well as the fMRI BOLD signal gives us an unprecedented opportunity to understand how the brain integrates all the information carried, at different spatial and temporal scales, by single neurons and by widespread networks. Yet progress in understanding how neural populations process information has been limited by a lack of analysis methods capable of comparing and merging the different types of information carried by different neural signals, and by the lack of realistic computational models of how integration of information can be achieved. Over the last 8 years, we have started the development of mathematical analysis and modeling tools to address such questions. Currently Stefano Panzeri, our students and I have 18 published and 4 in press papers on various aspects of signal nature and propagation, including a study on volume conduction, e.g. (Belitski, Gretton et al. 2008, Mazzoni, Panzeri et al. 2008, Montemurro, Rasch et al. 2008, Kayser, Montemurro et al. 2009, Belitski, Panzeri et al. 2010, Besserve, Scholkopf et al. 2010, Panzeri, Brunel et al. 2010).
In vivo measurement of cortical impedance spectrum in monkeys: Implications for signal propagation
Logothetis, N. K., Kayser, C., & Oeltermann, A. (2007). Neuron 55(5), 809-823
 To combine insights obtained from electric field potentials (LFPs) and neuronal spiking activity (MILIA) we need a better understanding of the relative spatial summation of these indices of neuronal activity. Compared to MUA, the LFP has greater spatial coherence, resulting in lower spatial specificity and lower stimulus selectivity. A differential propagation of low- and high-frequency electric signals supposedly underlies this phenomenon, which could result from cortical tissue specifically attenuating higher frequencies, i.e., from a frequency-dependent impedance spectrum. Here we directly measure the cortical impedance spectrum in vivo in monkey primary visual cortex. Our results show that impedance is independent of frequency, is homogeneous and tangentially isotropic within gray matter, and can be theoretically predicted assuming a pure-resistive conductor. We propose that the spatial summation of LFP and MUA is determined by the size of these signals' generators and the nature of neural events underlying them, rather than by biophysical properties of gray matter (Logothetis, Kayser et al. 2007).
To combine insights obtained from electric field potentials (LFPs) and neuronal spiking activity (MILIA) we need a better understanding of the relative spatial summation of these indices of neuronal activity. Compared to MUA, the LFP has greater spatial coherence, resulting in lower spatial specificity and lower stimulus selectivity. A differential propagation of low- and high-frequency electric signals supposedly underlies this phenomenon, which could result from cortical tissue specifically attenuating higher frequencies, i.e., from a frequency-dependent impedance spectrum. Here we directly measure the cortical impedance spectrum in vivo in monkey primary visual cortex. Our results show that impedance is independent of frequency, is homogeneous and tangentially isotropic within gray matter, and can be theoretically predicted assuming a pure-resistive conductor. We propose that the spatial summation of LFP and MUA is determined by the size of these signals' generators and the nature of neural events underlying them, rather than by biophysical properties of gray matter (Logothetis, Kayser et al. 2007).
A combined MRI and histology atlas of the rhesus monkey brain
Saleem, K. S., & Logothetis, N. K. (2006) Amsterdam, NY, Tokyo: Academic Press, Elsevier
 This atlas maps the detailed architectonic subdivisions of the cortical and subcortical areas in the macaque monkey brain using high-resolution magnetic resonance (MR) images and the corresponding histology sections in the same animal. It presents the detailed mapping of the architectonic areas in the horizontal plane of sections with reference to the MRI that had not been reported previously in macaque monkeys. The second part of the atlas presents the coronal plane using the same technique. A third part allows the quick identification of several important cortical and subcortical areas (in all, around 30 areas) in horizontal, coronal and sagittal MR images. This atlas is unlike anything else available as it includes and compares each section to imaging data. This is a significant step forward, because the vast majority of research in the field now routinely works with fMRI images (Saleem and Logothetis 2006).
This atlas maps the detailed architectonic subdivisions of the cortical and subcortical areas in the macaque monkey brain using high-resolution magnetic resonance (MR) images and the corresponding histology sections in the same animal. It presents the detailed mapping of the architectonic areas in the horizontal plane of sections with reference to the MRI that had not been reported previously in macaque monkeys. The second part of the atlas presents the coronal plane using the same technique. A third part allows the quick identification of several important cortical and subcortical areas (in all, around 30 areas) in horizontal, coronal and sagittal MR images. This atlas is unlike anything else available as it includes and compares each section to imaging data. This is a significant step forward, because the vast majority of research in the field now routinely works with fMRI images (Saleem and Logothetis 2006).
First findings related to the effects of DES (used also in patients) on large-scale networks
Our studies provide first in vivo measurements of brain-conductivity, and subsequently mapped the effects of electrical stimulation on the entire brain (Neuron and Nature-Neuroscience publications). Although my main interest is in basic research, I am very aware of the fact that such findings may be critical for clinical applications. Deep Brain Stimulation (DBS), for example, is used for the treatment of Parkinson’s’ disease (PD). Germany has the largest number of prevalent cases of PD of any country in Europe, with 150 cases per 100,000 inhabitants and 1,800 cases per 100,000 persons over the age of 65 years. The lives of tens of thousands of Parkinson’s patients have been transformed by DBS. Yet, 40% percent of all DBS-treated patients experience a multitude of serious adverse events, including nervous system and psychiatric disorders. In part the problems originate from the fact that the microstimulation methodology still needs to overcome two fundamental problems: (a) we do not always know exactly what is being stimulated when we pass currents through the tissue, and (b) stimulation causes activation in a large number of areas even outside the stimulation site, making it difficult to isolate and evaluate the behavioral effects of the stimulated area itself. The methodology we developed shows some of the potential problems during microstimulation and it is now being optimized in collaboration with the University of Tübingen for application in human DBS stimulation.
Introduction and first application of NET-fMRI in studies related to memory consolidation
Over several years recordings in different visual areas during the reported alternation of the animal’s perception had yielded novel and interesting findings, but to an increasing extent they had also indicated the complexity of the neural (perceptual) system, and perhaps even the inadequate “perceptron” role of single cells or small cellular populations. I gradually became convinced that the combination of behavioral studies with single or multiple unit recordings would never provide solid and translatable information at the systems level. While such studies are of immense value for learning basic physiological facts about single cells and small populations, they fall short of addressing system-behavior concepts. Undoubtedly, such approaches are still indispensable, and they will certainly be employed for many years to come, but I felt nevertheless that the time was ripe for the next step: the concurrent study of components and networks.
After several years the development and optimization our methods permitted the employment of a novel methodology which we call neural-event-triggered fMRI (NET-fMRI) in order to physiologically record neural events in one or more structures and used them for event-triggered imaging with various fMRI methodologies. For example, we have recently published a Nature paper (2012, more submitted currently) where a have recorded the hippocampal fast oscillations (ripples), occurring during deep-sleep and considered to be part of the mechanism underlying the consolidation of memories, in monkeys and use them as events to align and average the time courses of brain activations.
NET-fMRI revealed interesting and utterly unexpected patterns of up- and down-regulation of whole-brain activity at the times of ripple-occurrence. If the hippocampal-cortical interactions, reported to occur during the ripple-events are indeed related to memory consolidation, then the observed down-regulations – primarily confined in sensory thalamus and various structures related to procedural memory or paradoxical-sleep (with rapid eye movements) – likely reflect optimization of information transfer between various memory-related subsystems. In such a complex system, events like ripples are certainly not effectors but rather indicators of changes in the state widespread networks. States in turn, may depend on a large number of variables (e.g. activity changes in individual structures, or changes in inter-structure correlations), a subset of which will be characterized following intensive experimentation in the future. By combining the measurements afforded with NET-fMRI with animal behavior and the mathematics of dynamic systems, one can express the experimental outcomes as a partially ordered sequence of system-states; Such sequences may indeed provide information related to memory and cognition in general. The NET-fMRI methodology is unique worldwide with several US universities eagerly trying to transfer our technology to their institutions. The novelty and impact of the work has been commented several times; see example by Buzsaki.
Synthesis of Smart Contrast Agents
 Last but not least, we have now established excellent technical conditions for de novo synthesis of smart contrast agents (SCA).These are molecules whose relaxivity is a function of the concentration of a useful ion or molecule, usually called the target, e.g. a neurotransmitter of ions such as sodium and calcium. The term “relaxivity” denotes the ability of a contrast agent to shorten the relaxation time of nearby water protons. The higher the relaxivity is, the shorter the proton relaxation time. Despite the expected initial difficulties and the fact that chemistry is not the primary focus of my laboratory, we have been able to synthesize and characterize more than 30 potential SCA using gadolinium or europium as paramagnetic ions. A few of the synthesized SCA were sensitive to pH and others exhibited remarkable sensitivity towards Ca2+; the physicochemical characteristics of the latter were considerably better than those of any other currently available Ca2+-sensitive MR probe. All synthesis and characterization results have now been published in more than 16 in peer-reviewed chemistry journals, with another 5 papers currently under review. We have already tested the synthesized molecules in artificial CSF, extracellular matrix (ECM), and ultimately within the brain, and we are currently studying the biodistribution and wash-out of these agents both experimentally and with simulated diffusion studies. At the same time pulse sequences, e.g. Look-locker method, are optimized and further developed to separate BOLD and inflow effects from the actual intensity-changes due to the SCA-relaxivity changes (Mishra, Pfeuffer et al. 2006, Mamedov, Mishra et al. 2007, Angelovski, Fouskova et al. 2008, Mishra, Fouskova et al. 2008, Mamedov, Taborsky et al. 2009, Angelovski, Chauvin et al. 2010, Mamedov, Canals et al. 2010, Mamedov, Parac-Vogt et al. 2010, Mishra, Dhingra et al. 2010, Mishra, Schuez et al. 2011).
Last but not least, we have now established excellent technical conditions for de novo synthesis of smart contrast agents (SCA).These are molecules whose relaxivity is a function of the concentration of a useful ion or molecule, usually called the target, e.g. a neurotransmitter of ions such as sodium and calcium. The term “relaxivity” denotes the ability of a contrast agent to shorten the relaxation time of nearby water protons. The higher the relaxivity is, the shorter the proton relaxation time. Despite the expected initial difficulties and the fact that chemistry is not the primary focus of my laboratory, we have been able to synthesize and characterize more than 30 potential SCA using gadolinium or europium as paramagnetic ions. A few of the synthesized SCA were sensitive to pH and others exhibited remarkable sensitivity towards Ca2+; the physicochemical characteristics of the latter were considerably better than those of any other currently available Ca2+-sensitive MR probe. All synthesis and characterization results have now been published in more than 16 in peer-reviewed chemistry journals, with another 5 papers currently under review. We have already tested the synthesized molecules in artificial CSF, extracellular matrix (ECM), and ultimately within the brain, and we are currently studying the biodistribution and wash-out of these agents both experimentally and with simulated diffusion studies. At the same time pulse sequences, e.g. Look-locker method, are optimized and further developed to separate BOLD and inflow effects from the actual intensity-changes due to the SCA-relaxivity changes (Mishra, Pfeuffer et al. 2006, Mamedov, Mishra et al. 2007, Angelovski, Fouskova et al. 2008, Mishra, Fouskova et al. 2008, Mamedov, Taborsky et al. 2009, Angelovski, Chauvin et al. 2010, Mamedov, Canals et al. 2010, Mamedov, Parac-Vogt et al. 2010, Mishra, Dhingra et al. 2010, Mishra, Schuez et al. 2011).
Selected Reviews
What we can do and what we cannot do with fMRI
Logothetis NK (2008). Nature 453:869-878.
Functional magnetic resonance imaging (fMRI) is currently the mainstay of neuroimaging in cognitive neuroscience. Advances in scanner technology, image acquisition protocols, experimental design, and analysis methods promise to push forward fMRI from mere cartography to the true study of brain organization. However, numerous fundamental questions concerning the interpretation of fMRI data remain, as the conclusions drawn often ignore the actual limitations of the methodology. Here I give an overview of the current state of fMRI, and draw on neuroimaging and physiological data to present our current understanding of the hemodynamic signals and the constraints they impose on neuroimaging data interpretation.
Visual competition
Blake R, Logothetis NK (2002) Nature Reviews Neuroscience 3:13-23.
“The most recent evidence supports a view of rivalry as a series of processes, each of which is implemented by neural mechanisms at different levels of the visual hierarchy. Although unanswered questions remain, this view of rivalry might allow us to resolve some of the controversies and apparent contradictions that have emerged from its study.
 ... Visual information associated with a dominant stimulus flows uninterrupted throughout the visual pathways, triggering the normal complex of feedback connections, and making neural contact with all those processes that signal the semantic and affective connotations of a visual object or event. And however attention influences perception of a visual scene, it can likewise influence the perception of a dominant stimulus. The same cannot be said for a suppressed stimulus, however. VER, fMRI, MEG and single-unit studies all point to potent disruptions in neural processing during suppression phases of rivalry. Although controversial issues remain to be resolved, the emerging idea that rivalry involves multiple, distributed processes offers a very promising means to reconcile conflict in the rivalry literature.” (Blake and Logothetis 2002)
... Visual information associated with a dominant stimulus flows uninterrupted throughout the visual pathways, triggering the normal complex of feedback connections, and making neural contact with all those processes that signal the semantic and affective connotations of a visual object or event. And however attention influences perception of a visual scene, it can likewise influence the perception of a dominant stimulus. The same cannot be said for a suppressed stimulus, however. VER, fMRI, MEG and single-unit studies all point to potent disruptions in neural processing during suppression phases of rivalry. Although controversial issues remain to be resolved, the emerging idea that rivalry involves multiple, distributed processes offers a very promising means to reconcile conflict in the rivalry literature.” (Blake and Logothetis 2002)
Our work on rivalry was also published repeatedly in Scientific American (1999, 2006) and other SciAm books and chapters, and I have been told by the SciAm editors that it has been translated into over 30 languages. The 2006 article started with the words: “In their search for the mind, scientists are focusing on visual perception—how we interpret what we see.”, e.g. (Logothetis 1999)
fMRI and its interpretations: an illustration on directional selectivity in area V5/MT
Bartels, A., Logothetis, N. K., & Moutoussis, K. (2008) Trends in Neurosciences, 31(9), 444-453.
 fMRI is a tool that allows us to study brain function noninvasively. It can reliably identify sites of neural involvement for a given task. However, to what extent can fMRI signals be related to measurements obtained with electrophysiology? Can the blood-oxygen-level-dependent signal be interpreted as spatially pooled spiking activity? Here we combine knowledge from neurovascular coupling, functional imaging and neurophysiology to discuss whether fMRI has succeeded in demonstrating one of the most established functional properties in the visual brain, namely directional selectivity in the motion-processing region V5/MT+. We also discuss differences between fMRI and electrophysiology with respect to their sensitivity to distinct physiological processes. We conclude that fMRI constitutes a complement, not a poor-resolution substitute, to invasive techniques, and that it deserves interpretations that acknowledge its status as a separate signal.
fMRI is a tool that allows us to study brain function noninvasively. It can reliably identify sites of neural involvement for a given task. However, to what extent can fMRI signals be related to measurements obtained with electrophysiology? Can the blood-oxygen-level-dependent signal be interpreted as spatially pooled spiking activity? Here we combine knowledge from neurovascular coupling, functional imaging and neurophysiology to discuss whether fMRI has succeeded in demonstrating one of the most established functional properties in the visual brain, namely directional selectivity in the motion-processing region V5/MT+. We also discuss differences between fMRI and electrophysiology with respect to their sensitivity to distinct physiological processes. We conclude that fMRI constitutes a complement, not a poor-resolution substitute, to invasive techniques, and that it deserves interpretations that acknowledge its status as a separate signal.
Last but not least, I am including here two representative commentaries, mostly because they are representative of my own way of thinking in science. One of them is related to neurovascular coupling, and the other on the somewhat opportunistic use of new methodologies.
References
Blake, R. and N. K. Logothetis (2002). "Visual competition."Nature Reviews Neuroscience3(1): 13-23.
Blake, R. R. (1989). "A Neural Theory of Binocular Rivalry."Psychological Review96: 145-167.
Logothetis, N. K. (1990). "Subjective Perception - Reply."Science Magazine247(4943): 727.
Logothetis, N. K. (1999). Vision: A window on consciousness.Scientific American.281:68-75.