Animal welfare, the long-term physical and psychological well-being of non-human beings, is central to the animal research concept at ICPBR. Everyone at the institute working and interacting with animals, from the researchers and the veterinarians to the animal caretakers and the technicians, is keenly aware of the great responsibility that comes with conducting animal experiments. We see it as our ethical, moral, and human responsibility to provide the highest level of animal welfare to the animals in our care and treat each individual with the highest level of respect and dignity.
To establish and maintain high animal welfare standards, we follow internationally recognised and widely established practices like the 3R and 5F principles. The 3R principle was introduced in 1959 by Russel and Burch to remove inhumanity in the context of experimental techniques. The three Rs stand for Replacement, Reduction, and Refinement. The National Centre for the Replacement Refinement and Reduction of Animals in Research (NC3Rs) defines these terms the following way: Replacement is "accelerating the development and use of predictive and robust models and tools, based on the latest science and technologies, to address important scientific questions without the use of animals." Reduction refers to "appropriately designed and analysed animal experiments that are robust and reproducible, and truly add to the knowledge base." Refinement means "advancing research animal welfare by exploiting the latest in vivo technologies and by improving understanding of the impact of welfare on scientific outcomes."
At ICPBR, we are using the lowest sentient species possible to answer our research questions (replacement). However, due to the nature of the questions and the quest to study and understand higher brain functions, the species of choice are often non-human primates and rodents.
We use statistical and mathematical approaches to determine the smallest feasible number of animals that will yield meaningful results before an experimental series is started and, by this, can reduce the number of animals as much as possible (reduction). Constant quality control, optimisation, and further education help us to refine our research methods and to find ways to minimise potential suffering as much as possible (refinement).
The 5 F principle refers to the 5 freedoms any animal should be able to experience. These include Freedom from hunger, malnutrition and thirst; Freedom from fear and distress; Freedom from heat, stress or physical discomfort; Freedom from pain, injury and disease; and Freedom to express normal behaviour patterns. This principle was developed in 1965 and is a widely recognised principle to improve animal welfare. We strive to enable as many of these freedoms as possible for the animals housed at ICPBR.
Besides following these two well-established principles, we are taking more measures to ensure the optimal well-being and welfare of our animals and are unconditionally committed to improving our housing conditions and research techniques continuously.
Our non-human primates are socially housed in large enclosures with plenty of opportunities for climbing, foraging, and expressing species-typical behaviours. As naturally social animals with strong social interactions, hierarchies, and relationships, the social housing of non-human primates is one of the most essential measures. Having contact with conspecifics, interacting with cage mates, and building social structures help the animals stay healthy and reduce the formation of abnormal behaviour. The enclosures are enriched with trees, swings, toys, soft wood shavings, seating opportunities at different heights, and other enrichment.
The rodents at ICPBR are kept in appropriately sized cages, where feasible, in groups whose floors are covered with natural bedding materials, enabling the animals to engage in their natural burrowing and foraging behaviours. The cages are enriched with nesting materials and small shelters to provide a place for retreat and gnawing materials.
Animal welfare, animal well-being, enrichment plans, and animal surgeries and experiments are overseen by the institute's animal welfare officers (AWO). The number of well-trained AWOs is relative to the number of housed animals. The AWO further oversees the continuous education of all personnel with access to the animals, provides advanced training options, and ensures the correct handling of the animals.
Experiments in Animals Including Non-Human Primates
Introduction
The advancement of medicine, the expansion of knowledge about the mind and body, the innovation of treatments, the discovery of new drugs, and the improvement of human lives critically depend on animal research. While some progress can be made using computational models, simulations, calculations, and alternative methods, many key questions can only be answered by researching them in animals. Most animal research involves fruit flies, zebrafish, and rodents. However, when it comes to detailed questions about the human mind and body, and especially the human brain, no order of animals is more suited than primates. That includes humans themselves, but it also includes non-human primates. Due to their common ancestry and remaining similarities in anatomy and function, many non-human primate species are eminently suitable to investigate brain function, test medications, study development, and uncover underlying mechanisms of key processes in the body. However, these great similarities between non-human primates and humans make research on these beings ethically, morally, and technically challenging.
These challenges present themselves not only in conducting research but are also omnipresent in the discussions around it. While most of society knows animal research is expedient, necessary, and imperative, a smaller group of individuals believes strongly that animal research should not be conducted. Discussions between these groups are emotionally loaded, with strong convictions and moral beliefs. This makes the topic highly sensitive, but it cannot be a reason why it should not be discussed.
The next portion of the website shall address one of the most commonly voiced concerns of people who do not support animal research. Namely that animal research has never helped drive medical innovation.
This is, of course, a statement that can be easily refuted by just having a look at some of the inventions that directly resulted from animal research, like immunisation against polio, diphtheria, rubella, hepatitis, and Covid-19, defence against a huge number of microorganisms through broad-spectrum antibiotics and other anti-infective drugs, defence against pain through anaesthetics and analgesics, blood transfusions, intravenous feeding and medication, radiation therapies, open-heart surgery, therapy against drug addiction, coronary bypass, valve replacement, correction of congenital defects, medications for asthma, medications for the control of epileptic seizures, organ transplantation, kidney dialysis, medications to treat mental illness, cataract removal and artificial lens implants, microsurgery, treatment of injury to prevent paralysis, cochlear implants, and other potential prosthetic devices that might help completely paralysed patients. The list could be continued for pages and include medical advances in human as well as in veterinarian medicine. Besides the achievements of past research, ongoing research provides great hopes for a large number of people who have cancer, diabetes, Alzheimer's disease, various infectious diseases, cystic fibrosis, AIDS, long-Covid, devastating amyotrophic lateral sclerosis (A.L.S.), and many other diseases and disorders, for which our only hope is a better understanding of the body, mind, and brain.
In the next paragraphs, some past medical advances based on animal research are outlined and briefly described. Further, past and ongoing research with non-human primates is outlined to illustrate the benefits and the importance of animal and non-human primate research.
The Value of Animal Experiments
The following pages describe the contribution of animal experiments in discovering treatments for human diseases. Eight representative diseases and conditions were selected to illustrate animal research's invaluable contribution to their curing or treatment. A detailed account of more than 50 diseases can be found on the website of Understanding Animal Research [2] - Diseases & Research. However, not only humans but also animals greatly benefit from animal research. A detailed account describing the contribution of animal research in treating animal diseases and improving animal welfare in more than 15 examples can be found on the website Understanding Animal Research [3] – Veterinary Medicine. Further, the website [4] provides a comprehensive timeline depicting the greatest advances made in the last 150 years.
Anaesthetics
Anaesthetics, agents that can induce a reversible loss of consciousness, have greatly improved the well-being of patients and the outcome of surgeries. The concept of anaesthesia first started developing in the 18th century when it was seen that nitrous oxide could anaesthetise animals. After much testing and development, it was first used in dentistry. This anaesthesia was later replaced with the safer version, ether, which, however, still bore many dangers. In the late 19th century and the early 20th century, new and improved mixtures of gases were developed, as well as better systems to deliver them, all were tested on animals. So were anaesthetics developed later in the century, which were safer and non-flammable [5]. Until today, anaesthetics are being improved and help further improve the surgical experience both for the surgeon and the patient. To warrant safety and to explore different options, animal experiments are, now as ever, essential.
Cochlear Implants
More than 430 million people worldwide have disabling hearing loss (432 million adults and 34 million children), according to the World Health Organization (WHO), which is estimated to reach a staggering 700 million people by 2050 [6]. Hearing loss can have many different reasons, and while often it is acquired and comes with age, it can sometimes be congenital. One of the reasons for deafness (acquired or congenital) is a loss of the hair cells in the inner ear, limiting the cochlea's ability to transduce sound information from the environment to neural transmissions that the central nervous system can interpret as auditory sensations. Luckily, the nervous elements that transmit information from the cochlea to the brain usually remain functional, making it possible to stimulate the brain with a cochlear prosthesis. While a mere 60 years ago, deafness could not be treated, patients with a cochlear implant today can converse normally, even on the phone. The first stimulation of a human auditory nerve was done in 1957 [7], about 150 years after the first account of electrical stimulation of the auditory system was given by Alessandro Volta (1745 – 1827) [8]. Several years later, the first experimental implants were implanted into humans [9]. Subsequent developments and studies were carried out in cats, as the response from the unit's input was known, and by this, the spatial attenuation along the cochlea could be determined [10] and even today, cats and other animals are still used to optimise the technique further and improve the lives of people afflicted with deafness.
Diabetes
Diabetes is a chronic metabolic disease in which the production or function of insulin is disturbed. It used to be fatal, and while still one of the top ten leading causes of death worldwide [11], it has become treatable since insulin was isolated and purified in the 1920s after about 200 years of research.
Already in the 18th century, research into the causes of diabetes was conducted, and it was found in 1889 that removing the pancreas of a dog would lead to diabetes. Subsequently, insulin from dogs was isolated, but the injection of it into other organisms led to, besides a drop in blood sugar, a fatal rise in temperature. Only in early 1920 was the isolation more successful, after attempts were made to extract it from beef pancreas instead of dog pancreas. To test the concentration and activity of the isolated insulin, it was injected into rabbits, and their blood sugar levels were closely monitored.
The discovery of insulin was awarded the Nobel Prize in Physiology or Medicine in 1923 [12] and has saved countless lives since then. Today, insulin can be produced synthetically, though animal research is still needed to improve existing methods and to develop new approaches [13]. More details can be found in, e.g., [13] and [14].
Heart Surgery and Heart Transplants
Open-heart surgery, performed more than half a million times per year in the U.S. alone [15], is nowadays a routine procedure in high-income countries [16], thanks to extensive, decades-long animal research.
A milestone was reached when John Gibbon (1903 – 1973) invented the heart-lung machine [17]. In 1935 he performed surgery on a cat using an external pump to artificially replace the heart at the time of the operation. After 18 years of research and optimisation in other animals (e.g., dogs), the technique was used in humans [18]. The first successful operation was performed on an 18-year-old girl with right heart failure due to an atrial septal defect. A 45-minute operation was performed to correct the defect; the patient completely recovered and was recorded alive and well many years later [18]. However, at the time, the heart was still beating during the surgery, which made the procedure more difficult for the surgeon, who was presented with a moving target. Experiments using dogs, rabbits, and rats established that potassium citrate could be used to arrest the heart safely and that cold cardioplegia would protect it in this state [19].
Michael DeBakey (1908 – 2008), the "world's best-known surgeon" of his time [20], performed an estimated 60,000 operations over 70 years with exceptional skill and judgment [20]. He was the person to operate on world leaders, including presidents John F. Kennedy, Lyndon B. Johnson and Richard Nixon, Boris Yeltsin, The Duke of Windsor, King Hussein of Jordan, the Shah of Iran, and stars such as Marlene Dietrich. He pioneered pumps for heart-lung machines, coronary artery grafting, and blood vessel repair and was the first to repair an abdominal aortic aneurysm, perform a carotid endarterectomy, repair a dissecting aortic aneurysm, place an arterial patch graft, complete successful coronary artery bypass procedure, and to implant a left ventricular bypass pump [20].
DeBakey and his students' developments were based on animal experiments; they specifically worked on dogs and calves, and he passionately campaigned for animal experimentation. Further, he supported the Foundation for Medical Research, which promotes animal experiments, by not charging his rich clients but having them donate to this foundation.
 "While I was at the Baylor College of Medicine, I was honoured with the DeBakey Award for Excellence in Science for my monkey research, aiming at a better understanding of the neural mechanisms of visual perception. The award was presented to me by Michael DeBakey himself; I vividly remember his human warmth, his enthusiasm for our research and his great interest in brain science. The opportunity to meet such people has always been a great inspiration to me." – Nikos Logothetis
"While I was at the Baylor College of Medicine, I was honoured with the DeBakey Award for Excellence in Science for my monkey research, aiming at a better understanding of the neural mechanisms of visual perception. The award was presented to me by Michael DeBakey himself; I vividly remember his human warmth, his enthusiasm for our research and his great interest in brain science. The opportunity to meet such people has always been a great inspiration to me." – Nikos Logothetis
Hypertension
Hypertension, a higher-than-normal blood pressure, is a very common, serious medical condition that can severely impact the organs of a being and ultimately lead to death. Animal research has greatly helped develop treatments for this condition, making it a chronic but manageable disease that is not necessarily fatal anymore.
In the late 1940s, experiments on the nervous systems of cats and rabbits led to the discovery of the first drugs that were further improved upon in the years to come. Before that discovery, the adrenal glands were removed in attempts to treat high blood pressure, which had dire side effects and required the substitution of hormones for the rest of their life. In the 1960s, a new type of drug, beta-blockers, tested in rats, promised even better results in lowering blood pressure [21]. In the 1970s, further advancements were made, and a third type of drug was developed by conducting experiments in rats. Today, hypertension, if detected early enough, can be treated with different drugs, enabling the affected person to lead a normal and long life.
Infections and Diseases
Until the middle of the 19th century, the prevalent belief was that bodily derangements cause diseases and ailments. Treating those often did not lead to successful treatment of the ailment but rather to the patient's death. The belief system regarding the cause of diseases started to change with the work of Louis Pasteur (1822 – 1895) [22] and his contemporaries. Pasteur knew how drinks like wine and beer got spoiled by microorganisms and hypothesised that such organisms could be the cause of human or veterinarian diseases as well. As a model to test his hypothesis, he chose the cholera disease in chickens. He was able to isolate bacteria from the chicken's intestines through laborious and invasive research that could not have been conducted in humans, and he realised upon administering these bacteria to healthy chickens that the previously healthy chicken got sick. With this, he could prove the cause of some infections to be microorganisms. He later included rabbits and guinea pigs in his studies and found through more research that when the infectious cultures were administered later, the animals did not turn sick and were even immune to the disease. With this, he discovered the principle of immunisation. Scientists have further developed the principle; today, 25 diseases can be prevented using vaccines. According to the WHO, these are cholera, Covid-19, dengue fever, diphtheria, Haemophilus influenzae type b, hepatitis A, hepatitis B, hepatitis E, human papillomavirus, influenza, Japanese encephalitis, malaria, measles, meningococcal meningitis, mumps, pertussis, pneumococcal disease, poliomyelitis, rabies, rotavirus, rubella, tetanus, tick-borne encephalitis, tuberculosis, typhoid fever, varicella, yellow fever, [23]. Further, vaccines against enterotoxigenic escherichia coli, group B Streptococcus, herpes simplex virus, HIV-1, malaria, neisseria gonorrhoea, nontyphoidal salmonella disease, norovirus, paratyphoid fever, respiratory syncytial virus, schistosomiasis disease, shigella, group A streptococcus, tuberculosis, and improved influenza vaccines are in development [23].
Before Covid-19, The WHO estimated that immunisation efforts prevented about 4 – 5 million death per year [24]. An additional 20 million lives were saved by the Covid vaccine, according to estimations of scientists [25], and even more, can be saved in the future when new vaccination techniques like m-RNA vaccines become more widely available.
The discovery by Pasteur set other scientists on their path. A prominent example is the British surgeon Joseph Lister (1827 – 1912), who began, after hearing about Pasteur's findings, to sterilise his surgical instruments, sutures, and wound dressings with acid to lower infection rates during and after surgery. This lowering of surgical infections dramatically improves the survival rate of patients, and in combination with the aforementioned vaccine developments and the plethora of other inventions and discoveries that sprang from Pasteur's findings, the number of saved lives (human and animal) is countless.
Just like Pasteur's research, much of subsequent research was conducted in animals. And even Lister, who had publicly condemned the horrible practices of animal experimentalists in earlier years, replied, when asked by Queen Victoria herself, that "animal experiments had been essential to his work on asepsis and that restricting animal research would prevent discoveries that would benefit humankind" [26].
Kidney Transplants
Kidney transplants are the most performed organ transplant in humans today [27]. More than 25000 kidneys were transplanted in the U.S. in 2022 alone [28]. Worldwide, organ transplants reached more than 144000 annually (2021) [29]. These staggering numbers show the importance of medical achievements in this field of medical research.
Getting to these successful transplantations and being able to help all those people affected took many decades of dedicated, tedious animal research. While the idea of transplantation has been around for more than two millennia, success in the endeavour has only been consistently documented in the last 70 years [30]. The research conducted over time in this field involved animals at almost every step of the way. From suturing techniques of blood vessels to transplants of skin patches, tumours, and whole organs, to the discovery of the principles of immunology and the development of anti-rejection medications and immune suppressants, animals played a crucial role. While a detailed description of this long and difficult process would go beyond the scope of this essay, even if only the landmark achievements were mentioned, the importance of this branch of animal research is mirrored in the number of Nobel prizes awarded in this context. 5 prizes were awarded for transplantation progress and 14 more for immunology-related research [30, 31]. An excellent and detailed Historical Overview of Transplantation is available from Barker and Markmann [30], describing the role of animals in developing the lifesaving technique of organ transplantation.
Non-Human Primates in Basic and Translational Research
As described above, animal research has significantly impacted humankind's understanding and treatment of diseases. And while many questions can be answered using model organisms like rodents, flies, or fish, other questions rely on animal models closer to humans, like non-human primates. The following pages will describe how non-human primates have been used in basic and translational research to uncover and understand the underlying mechanisms of diseases, to develop and test treatment options and therapeutic interventions for humans, and to further the fundamental understanding of how the brain works.
Constraint-Induced Movement Therapy
Constraint-Induced Movement Therapy (CIMT), a method developed by Eduard Taub (*1970) in early 1990, is an important form of treatment that can help certain patients with damage to their brain or spinal cord after, e.g., a stroke to regain the use of affected limbs [32]. A "learned non-use" process often sets in after patients get discouraged by the difficulty of using the affected limb after the event that damaged their central nervous system. During CIMT, the non-affected limb is a constraint, so the patient is required to use the affected limb. It was shown that regaining function is possible by developing new neuronal pathways in the brain [33].
Taub used non-human primates for his experiments and deafferented their limbs. Even though they could not feel them anymore, they could use them again if motivated by electric stimulation or starvation.
After the experiments were concluded, one animal was studied in detail to see if changes in the brain had occurred. For this, electrodes were placed in the brain under anaesthesia, and hundreds of recordings were taken, revealing what the Laboratory Primate Newsletter called an "unprecedented degree of reorganisation of the sensory cortex. An 8-10-millimetre wide area that would normally receive input from the hand was found to have filled in with input from the face". Scientists also discovered an unpredicted change in the structure of the thalamus, apparently caused by progressive nerve degeneration through the severed dorsal root ganglia and the dorsal columns all the way to the thalamus. Based in part on this work, Taub went on to develop novel physical therapy techniques to help stroke victims and those with other forms of brain injury regain the use of affected limbs. The American Stroke Association calls Taub's therapy, CIMT, "at the forefront of a revolution" in treating stroke survivors [34].
Studies have since revealed that the brain is indeed able to reorganise and rehabilitate function to a certain extent, a concept which is now used to help patients regain functions in their affected limbs [35].
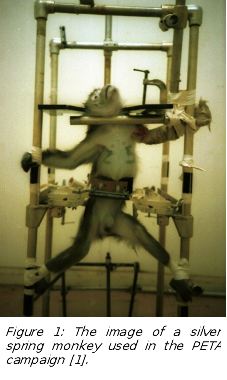 It is worth mentioning that Taub – despite the importance of his research - was sued by PETA (People for the Ethical Treatment of Animals). Many people saw the photographs of the Silver Spring Monkeys portrayed as crucified (Figure 1), but they were almost certainly unaware that the Silver Spring monkey photograph was staged by the animal-rights caretakers [1]. The police temporarily housed the confiscated monkeys in the basement of a house belonging to a member of PETA. The animals were then reported stolen, but after it became clear that Taub could not be prosecuted without the monkeys as evidence, they "reappeared" and were returned to Taub before being transferred to an NIH (National Institute of Health) facility. No one was ever charged with the theft of court evidence, but it was reported they had been transferred to a refuge in Gainesville, Florida. In January 1990, the court allowed a group of researchers from the NIH to conduct a terminal experiment on one of the monkeys who had become ill.
It is worth mentioning that Taub – despite the importance of his research - was sued by PETA (People for the Ethical Treatment of Animals). Many people saw the photographs of the Silver Spring Monkeys portrayed as crucified (Figure 1), but they were almost certainly unaware that the Silver Spring monkey photograph was staged by the animal-rights caretakers [1]. The police temporarily housed the confiscated monkeys in the basement of a house belonging to a member of PETA. The animals were then reported stolen, but after it became clear that Taub could not be prosecuted without the monkeys as evidence, they "reappeared" and were returned to Taub before being transferred to an NIH (National Institute of Health) facility. No one was ever charged with the theft of court evidence, but it was reported they had been transferred to a refuge in Gainesville, Florida. In January 1990, the court allowed a group of researchers from the NIH to conduct a terminal experiment on one of the monkeys who had become ill.
Locked-in Syndrome and Brain-Computer Interfaces
The locked-in syndrome is a rare neurological disorder in which a patient's body is almost fully paralysed and has lost the ability to detect sensory input. The vertical eye muscles, hearing, and cognition are often still intact (sometimes some other motor functions might be residual, and sometimes not even vertical eye movements or blinking are preserved), leaving these patients with fully functioning brains locked into unmoving bodies. The reason for this is damage to the brain stem or midbrain, caused by, e.g., traumatic brain injury, stroke, circulatory system diseases, diseases that destroy the myelin sheath (a protective covering surrounding nerve cells), or medication overdose [36, 37]. While the locked-in state can be reversed in some cases by removing the cause (e.g., a stroke, bleeding, tumour), no treatment is available for patients with chronic lesions in the crucial areas. While they can survive with medical aid like ventilation, fluid therapy, and other basic measures, they cannot communicate with the outside world.
However, as these patients still have functioning brains, their brain waves can be deciphered, and the code used to interpret their thoughts. The first studies to understand the brain waves recorded with an EEG were made in 1970 on cats. Around that time also, the first brain-computer interface (BCI) was described. This is a technique in which brain signals (EEG or others) are used to control a machine. In the case of, e.g. paralysed patients, a machine controlled by the thoughts can be used to control an external mechanic limb to conduct simple movements their bodies cannot perform [38]. While crude forms of this have been available after much animal research, a greater fundamental understanding of the brain is required to optimise this method and make it available for more patients. According to Kawala-Sterniuk and her colleagues, advances must be made in signal-acquisition hardware, BCI validation and dissemination, and reliability. For this, more animal research, especially in non-human primates, is needed to give hope to people relying on these methods to improve their lives, e.g., patients suffering from A.L.S. (Amyotrophic Lateral Sclerosis), cerebral palsy, brainstem stroke, spinal cord injuries, muscular dystrophies, chronic peripheral neuropathies, or psychiatric disorders [38].
A success story
In 2012, Hochberg and colleagues published that two patients with long-standing paralysis could control the reaching and grasping actions of a robotic arm using a neural-interface system known as BrainGate [39]. BrainGate consists of sensors, decoders, and external devices, such as computers, wheelchairs or prosthetic/robotic limbs. Remarkably, one of the patients could even drink from a bottle using the robotic arm, something she had not been able to do with her limb since a stroke nearly 15 years ago [39]. The results of the above pilot study drew directly on previous neural-interface demonstrations in monkeys (e.g., from Eb Fetz [40], Niels Birbaumer [41], or John Donoghue's lab [42]) and decades of basic research into the control of arm movements.
Deep Brain Stimulation
Parkinson's disease, the second most common neurodegenerative disorder in the U.S., affects the central nervous system and is a chronic and progressive disease. About 500000 Americans are diagnosed each year. Adding the undiagnosed cases, the estimation of afflicted people lies around 1 million in the U.S. alone [43]. Worldwide, the number of people living with the diagnosed disease exceeds 10 million [44].
Characteristic symptoms of Parkinson's are tremors, bradykinesia, hypokinesia, rigidity, and postural instability [44], which progress and worsen over time. Caused by the death of neurons in the substantia nigra and with this a dopamine deficit (the concrete underlying mechanisms are unknown), the disease cannot be healed, as there is currently no method available to restore lost brain tissue. Depending on the progression of the disease, symptomatic treatments like medications, physical, occupational, and speech therapies, a healthy diet, massages, and exercises can help to relieve the symptoms [45]. One of the most impressive interventions for patients with treatment-resistant Parkinson's disease is deep brain stimulation (DBS). For this, electrodes are implanted deep into the subthalamic nucleus to act as pacemakers, and by passing a current through them, they can activate specific brain areas and eliminate or reduce tremors, the severe dyskinesias that blight the lives of severely affected patients. DBS can also treat other disorders like drug-resistant diseases, including obesity, obsessions, schizophrenia, and various forms of depression.
Developed in animals such as non-human primates, this therapeutic approach has improved the lives of more than 160,000 patients (2019) [46]. With a long history of brain stimulation starting in the early 19th century [47], the first therapeutic deep brain stimulations were conducted in the late 1930ies to treat psychosis [48]. The first treatment of motor disorders occurred in the early 1960ies [49]. In the following decades, the method was further studied and optimised in animals (e.g., cats, monkeys, chimpanzees, gibbons, bulls, etc.) [50-53]. Since the 1990ies, the methods have been more and more used in Parkinson's patients, with developments from stimulating the Thalamus [54] and the globus pallidus [55] to the subthalamic nucleus[56], [57], [47].
While DBS can greatly help patients, it is also a dangerous procedure that can lead to side effects like surgical site infection, nervous system disorders, psychiatric disorders, device-related complications, intracerebral haemorrhages, infectious brain disease, or cardiac disorders [58, 59]. To reduce these serious complications for patients in the future, a better understanding of the general principles of electrical stimulation, particularly its spatial extent and remote effects, must still be acquired. Further, it should be researched when is the best time to start this therapy, in which patients it is most promising, or if changing the location or adding more electrodes can further improve the method [47]. To answer these questions and to improve the quality of care for these patients, more animal research is needed to find safe and improved ways.
Examples of patient interviews and synoptic descriptions of surgeries
The videos in the following links show the effects of DBS on two Parkinson's patients (1, 2). The third video (3) shows the surgical procedure for the implantation of a DBS device. The videos illustrate the great improvement in the patients' lives due to DBS and the reality of surgeries to achieve this.
2) The effects of D.B.S. on the motor symptoms of Parkinson's Disease
3) Patient Guide to Deep Brain Stimulation (D.B.S.) Surgery, Mayfield Clinic
Alzheimer's Disease
According to the WHO, more than 55 million people worldwide suffer from dementia. 60 – 70 % of those have Alzheimer's disease [60]. Humans have documented dementia for more than 4000 years. Historically, reports of declining memory function in ageing people have been found, though the term dementia was only used in the medical context in the 18th century [61]. Since then, research efforts in this field have further expanded. In 1910 dementia was first categorised into senile dementia and presenile dementia, and shortly after, presenile dementia was named Alzheimer's disease in honour of the researcher Alois Alzheimer, who discovered the characteristic pathological features [62]. Alzheimer's is characterised by a loss of brain tissue due to the accumulation of amyloid plaques and neurofibrillary tangles [63]. This loss, due to the disruption of vital processes within the neuron, often starts in brain areas associated with memory, like the entorhinal cortex and hippocampus, leading to the well-known symptom of forgetting recent events. With the progression of this neurodegenerative disease, other brain areas like the cerebral cortex (mainly areas for language, reasoning, and social behaviour) are affected [64].
While the underlying pathological changes within the brain were discovered more than one hundred years ago, the underlying primary causes of this disease are still unknown. Further, no cure has been found yet [63]. However, extensive research involving animal models and cell cultures has furthered the understanding of the disease and helped develop medications. Some drugs can bring symptomatic relief, like cholinesterase inhibitors [65], that can aid in the control or reduction of certain cognitive and behavioural symptoms. The first drug to target not only the symptoms but the underlying pathology was developed in animals (e.g., [66, 67]) and is now being tested in humans [68]. This antibody shall help to reduce amyloid plaques, the lesion-causing proteins.
Much of the research concerning Alzheimer's was conducted in mice. However, the translational power from mice to humans is not as large as the translational power from non-human primates to humans. Therefore, Alzheimer models in non-human primates have been and are currently being developed (e.g., [69-71]). The hope is that with better models, better therapeutic interventions can be discovered and tested.
Transgenic Non-Human Primates
As illustrated in the examples above, non-human primates are still essential in research to further humankind's knowledge and to save and improve the lives of millions of people and animals. In the last decade, a new and exciting avenue has opened up to scientists in non-human primate research: transgenic non-human primates. While other species (e.g., rodents, fruit flies) have had genes inserted into their genomes by scientists since the early 1980ies [72], the methodology in non-human primates was more challenging, and the results less stable. Only in the new century, the first transgenic non-human primate was born [73]. Since then, the development sped up, and today transgenic non-human primates (different macaque species) are available to model several different disorders (e.g., Rett Syndrome [74], autism [75], sleep and psychiatric disorders [76], Huntington's [77] (see below)).
While the use of transgenic non-human primates is controversial [78], and researchers are aware of this [79], it is essential. Strict implementation of rules and regulations, principles like the 3R principle [80], improvement of methods and techniques, and strict controls and oversight allow for this challenging research to be conducted humanely.
Huntington's Disease
Huntington's is a neurodegenerative disease that impairs the basal ganglia, a brain structure that plays a key role in motor and non-motor functions [81]. Patients afflicted with the disease exhibit progressively worsening motor symptoms like impairment of eye saccades [82], seizures, or rigidity [83, 84], cognitive symptoms like cognitive decline [85], dementia with visuospatial memory and executive function impairments [86], or memory recall [87], and/or psychotic symptoms like behavioural disorders, irritability, or aggression [88].
So far, patients can only receive symptomatic relief, as no cure for the disease is available. However, research is ongoing, and new methods are being developed trying to help understand the disease and potential treatment options better. In the late 21st century, scientists started to develop transgenic mouse models that replicated some of the genetic mutations (e.g., [89, 90]. While these models greatly helped with the basic understanding of the disease, they do not model all aspects well. Therefore, transgenic non-human primates with gene mutations were developed and observed [91]. These animals can be studied throughout their life, and more aspects of the disease can be uncovered. The knowledge gained from transgenic non-human primates can be combined with studies in cells, mice, and humans, creating a more holistic understanding of the disease, its pathogenesis, and potential treatment options [81].
Brain Tumours
Glioblastomas are tumours that form out of astrocytes, a subtype of the glial cells within the brain. Glioblastoma is the most common type of brain cancer, with about 14000 new diagnoses annually in the U.S. alone [87]. Due to its rapid growth, it is a very lethal tumour, and less than 5 – 15 % of patients survive 5 years following diagnosis [92, 93]. Treatment of this aggressive cancer has proven difficult. Even if, shortly after diagnosis, surgery is performed in which all visible parts of the tumour are taken out, it usually does not stop the growth, it only slows it down. Often such surgery is followed by radiation, but this, too, does not cure cancer. Treatment is notoriously difficult given the location within the brain and the protective blood-brain barrier that prevents most chemicals and medications from entering the brain [93]. However, recently, a great breakthrough was reported that brings hope to patients suffering from this disease [94]: The immune system cannot distinguish the cancerous cells from healthy cells and can not attack the cancer cells. But if the tumour cells were marked by something the immune system could recognise, it could attack and destroy the cells. By introducing a modified poliovirus into the brain, the virus can attach itself to the tumour cells. The immune system then detects the virus, and the cells can be destroyed. More than 18 years of research were needed to develop and optimise this method so that it can now be tested in humans. The most difficult part was introducing the virus into the tumour cells, but experiments in animals, including non-human primates, enabled scientists to find a safe and effective way [95]. The methods are currently being tested in humans and have been found to have an increased survival rate [94, 96]. However, glioblastomas are still a devastating and lethal disease and more research in animals and other models are needed to increase the survival rate of afflicted patients.
Covid-19 Vaccine
At the end of 2019, the world was brought to a halt by an unexpected pandemic. Covid-19 emerged and killed millions of people. While countries were reacting with hygiene measures, mask rules, quarantine, and social distancing, researchers were trying franticly to find a cure and a vaccine for this new, vastly spreading virus. In August 2021, the FDA approved the first-ever Covid-19 vaccination [97]. With less than two years from virus breakout to FDA approval, the development of this vaccine was by far the fastest in history. But how was this possible?
The newly developed Covid-19 vaccine was an m-RNA vaccine, the first approved one of its kind, but not the first to develop. mRNA, or messenger RNA, was discovered in the early 1960ies. In the mid 1960ies, the first liposomes, fatty bubbles composed of lipid molecules, were produced. These liposomes can envelop m-RNA and enable transport into the cells. The first experiments with this combination were done in the late 1970ies after 10 years of other experiments with liposomes. About a decade later, m-RNA could be synthesised in the lab, and in the early 1980ies, synthetic m-RNA was enveloped in liposomes and delivered into frog embryos and, shortly after that, into mice. From thereon, m-RNA was used in animals trying to develop, e.g., cancer or influenza vaccines in mice and other treatments in rats. Many difficulties remained, and only in 2005 was it possible to develop the system in a way immune cells would not destroy it. In parallel, liposomes were continuously improved, and lipid nanoparticles were developed in 2005.
Further research with the combination of m-RNA and lipid nanoparticles led to testing a vaccine against rabies and influenza in mice after 2010. After 2015, the first drug using lipid nanoparticles was approved, and research continued for vaccines using m-RNA [98, 99]. After all this groundwork was laid, modifying the m-RNA to use it against Covid was possible and testing the new vaccine in animals could begin shortly after the DNA sequence of Covid-19 was published. After exhaustive tests in rodents and non-human primates, the vaccine was approved for human use, saving millions of lives.
Non-Human Primate Research in China
After a long period where China mainly supplied non-human primates to research labs worldwide, it has changed its position in the last decades and established itself as a leader in non-human primate research [100]. An abundance of non-human primates, an accepting society, support from the government, and a highly competitive, high-quality research environment enabled it to push non-human primate research to new levels. Due to difficulties conducting adequate non-human primate research in Europe and the U.S. (based on unacceptance in society and non-rational decisions by governments), many research groups at universities, institutes, and companies have outsourced their research and collaborated with Chinese scientists. Intensively studied fields include research on monkey embryo reprogramming, differentiation of macaque stem cells into neural progenitor cells, programmed expression of the non-human primate genome during embryonic development, comparative genomics, chronic hepatitis, diabetes, geriatrics, metabolic syndromes, L-DOPA-induced dyskinesias, Parkinson's disease, diabetes, or cardiovascular disease.
Standards regarding animal welfare and well-being are constantly raised, husbandry conditions improved, and research conditions adapted to international standards, making China an important player in advancing human knowledge.
References
1. Wikipedia. Silver Spring monkeys. 2023; Available from: https://en.wikipedia.org/wiki/Silver_Spring_monkeys.
2. Understanding Animal Research. Diseases & Research. 2014; Available from: https://www.animalresearch.info/en/medical-advances/diseases-research/.
3. Understanding Animal Research. Veterinary Medicine. 2018; Available from: https://www.animalresearch.info/en/medical-advances/veterinary-medicine/.
4. Understanding Animal Research. Medical discovery timeline. 2021; Available from: https://www.animalresearch.info/en/medical-advances/medical-discovery-timeline/.
5. Understanding Animal Research. Anaesthetics. 2015; Available from: https://www.animalresearch.info/en/medical-advances/diseases-research/anaesthetics/.
6. World Health Organisation. Deafness and hearing loss. 2023; Available from: https://www.who.int/news-room/fact-sheets/detail/deafness-and-hearing-loss.
7. Djourno, A. and C. Eyries, [Auditory prosthesis by means of a distant electrical stimulation of the sensory nerve with the use of an indwelt coiling]. Presse Med (1893), 1957. 65(63): p. 1417.
8. Asimov, I., Asimov's biographical encyclopedia of science and technology. 1971.
9. House, W.F. and J. Urban, Long term results of electrode implantation and electronic stimulation of the cochlea in man. Annals of Otology, Rhinology & Laryngology, 1973. 82(4): p. 504-517.
10. Merzenich, M.M., et al., Neural encoding of sound sensation evoked by electrical stimulation of the acoustic nerve. Ann Otol Rhinol Laryngol, 1973. 82(4): p. 486-503.
11. World Health Organisation. The top 10 causes of death. 2020; Available from: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death.
12. NobelPrize.org. The Nobel Prize in Physiology or Medicine 1923 2023; Available from: https://www.nobelprize.org/prizes/medicine/1923/summary/.
13. Understanding Animal Research. Diabetes. 2022; Available from: https://www.animalresearch.info/en/medical-advances/diseases-research/diabetes/.
14. Botting, J.H., Animals and Medicine. 2015, Cambridge: Open Book Publishers.
15. Cleveland Clinic. Heart Surgery 2022; Available from: https://my.clevelandclinic.org/health/treatments/17525-heart-surgery.
16. Vervoort, C., et al., Global cardiac surgery: Access to cardiac surgical care around the world. The Journal of Thoracic and Cardiovascular Surgery, 2020. 159(3): p. 987-996.e6.
17. DeBakey, M.E., John Gibbon and the heart-lung machine: a personal encounter and his import for cardiovascular surgery. The Annals of Thoracic Surgery, 2003. 76(6): p. S2188-S2194.
18. Bellis, M. Biography of John Heysham Gibbon Jr., Heart-Lung Machine Inventor. 2019; Available from: https://www.thoughtco.com/heart-lung-machine-john-heysham-gibbon-4072258.
19. Understanding Animal Research. History of the heart-lung machine. 2023; Available from: https://www.understandinganimalresearch.org.uk/why/human-diseases/history-of-the-heart-lung-machine.
20. National Library of Medicine. The Michael E. DeBakey Papers. 2019; Available from: https://profiles.nlm.nih.gov/spotlight/fj/feature/biographical.
21. Prichard, B.N., Hypotensive Action of Pronethalol. Br Med J, 1964. 1(5392): p. 1227-8.
22. Science History Institute. Louis Pasteur. 2017; Available from: https://www.sciencehistory.org/historical-profile/louis-pasteur.
23. World Health Organisation. Immunization, Vaccines and Biologicals. 2023; Available from: https://www.who.int/teams/immunization-vaccines-and-biologicals/diseases.
24. World Health Organisation. Immunization. 2019; Available from: https://www.who.int/news-room/facts-in-pictures/detail/immunization.
25. Watson, O.J., et al., Global impact of the first year of COVID-19 vaccination: a mathematical modelling study. The Lancet, Infectious Diseases, 2022. 22(9): p. 9.
26. Botting, J.H. and A.R. Morrison, Animal Research Is Vital to Medicine. Scientific American, 1997. 276(2): p. 83-85.
27. Statista. Estimated number of organ transplantations worldwide in 2021. 2023; Available from: https://www.statista.com/statistics/398645/global-estimation-of-organ-transplantations/.
28. UNOS. Fast Facts. 2023; Available from: https://unos.org/about/fast-facts/.
29. Global Observatory on Donations and Transplantation. The global database on donation and transplantation. 2023; Available from: https://www.transplant-observatory.org.
30. Barker, C.F. and J.F. Markmann, Historical overview of transplantation. Cold Spring Harb Perspect Med, 2013. 3(4): p. a014977.
31. NobelPrize.org. The Nobel Prize in Physiology or Medicine 2023; Available from: https://www.nobelprize.org/prizes/lists/all-nobel-laureates-in-physiology-or-medicine/.
32. Taub, E., CI therapy: A new rehabilitation technique for aphasia and motor disability after neurological injury. 2002.
33. Physiopedia. Constraint-Induced Movement Therapy (CIMT). 2023; Available from: https://www.physio-pedia.com/Constraint-Induced_Movement_Therapy_(CIMT)#cite_note-6.
34. Bani-Ahmed, A.A., Post-stroke motor recovery and cortical organization following Constraint-Induced Movement Therapies: a literature review. J Phys Ther Sci, 2019. 31(11): p. 950-959.
35. Taub, E., G. Uswatte, and V.W. Mark, The functional significance of cortical reorganization and the parallel development of CI therapy. Front Hum Neurosci, 2014. 8: p. 396.
36. National Institute of Neurological Disorders and Stroke. Locked-In Syndrome. 2023; Available from: https://www.ninds.nih.gov/health-information/disorders/locked-syndrome.
37. Das, J., K. Anosike, and R.M.D. Asuncion. Locked-in Syndrome. 2022; Available from: https://www.ncbi.nlm.nih.gov/books/NBK559026/.
38. Kawala-Sterniuk, A., et al., Summary of over Fifty Years with Brain-Computer Interfaces-A Review. Brain Sci, 2021. 11(1).
39. Hochberg, L.R., et al., Reach and grasp by people with tetraplegia using a neurally controlled robotic arm. Nature, 2012. 485(7398): p. 372-5.
40. Jackson, A., et al., The Neurochip BCI: towards a neural prosthesis for upper limb function. IEEE Trans Neural Syst Rehabil Eng, 2006. 14(2): p. 187-90.
41. Birbaumer, N., A.R. Murguialday, and L. Cohen, Brain-computer interface in paralysis. Curr Opin Neurol, 2008. 21(6): p. 634-8.
42. Bansal, A.K., et al., Decoding 3D reach and grasp from hybrid signals in motor and premotor cortices: spikes, multiunit activity, and local field potentials. J Neurophysiol, 2012. 107(5): p. 1337-55.
43. National Institute of Neurological Disorders and Stroke. Parkinson's Disease: Challenges, Progress, and Promise. 2023; Available from: https://www.ninds.nih.gov/current-research/focus-disorders/focus-parkinsons-disease-research/parkinsons-disease-challenges-progress-and-promise.
44. Parkinson's Foundation. Who has Parkinson's? 2022; Available from: https://www.parkinson.org/understanding-parkinsons/statistics.
45. National Institute on Aging. Parkinson’s Disease: Causes, Symptoms, and Treatments. 2022; Available from: https://www.nia.nih.gov/health/parkinsons-disease.
46. Lozano, A.M., et al., Deep brain stimulation: current challenges and future directions. Nat Rev Neurol, 2019. 15(3): p. 148-160.
47. Sironi, V.A., Origin and evolution of deep brain stimulation. Front Integr Neurosci, 2011. 5: p. 42.
48. Cerletti, U., L'electroshock. Riv. Sper. Freniat. Med Leg Alienment, 1940. 64: p. 209-310.
49. Bekhtereva, N., K. Grachev, and A. Orlova, Utilization of multiple electrodes implanted in the subcortical structure of the human brain for the treatment of hyperkinesis. Zhurnal nevropatologii i psikhiatrii imeni SS Korsakova (Moscow, Russia: 1952), 1963. 63: p. 3-8.
50. Delgado, J.M.R., H. Hamlin, and W.P. Chapman, Technique of intracranial electrode implacement for recording and stimulation and its possible therapeutic value in psychotic patients. Stereotactic and Functional Neurosurgery, 1952. 12(5-6): p. 315-319.
51. Hosobuchi, Y., J.E. Adams, and B. Rutkin, Chronic thalamic stimulation for the control of facial anesthesia dolorosa. Arch Neurol, 1973. 29(3): p. 158-61.
52. Mazars, G., L. Merienne, and C. Cioloca, [Treatment of certain types of pain with implantable thalamic stimulators]. Neurochirurgie, 1974. 20(2): p. 117-24.
53. Hassler, R., et al., EEG and clinical arousal induced by bilateral long-term stimulation of pallidal systems in traumatic vigil coma. Electroencephalogr Clin Neurophysiol, 1969. 27(7): p. 689-90.
54. Blond, S. and J. Siegfried, Thalamic stimulation for the treatment of tremor and other movement disorders. Acta Neurochir Suppl (Wien), 1991. 52: p. 109-11.
55. Laitinen, L.V. and A.T.H. Bergenheim, M. I., Leksell's posteroventral pallidotomy in the treatment of Parkinson's disease. Journal of neurosurgery, 1992. 76(1): p. 53-61.
56. Pollak, P., et al., Effets de la stimulation du noyau sous-thalamique dans la maladie de Parkinson. Revue Neurologique (Paris), 1993. 149(3): p. 175-176.
57. Yu, H. and J.S. Neimat, The treatment of movement disorders by deep brain stimulation. Neurotherapeutics, 2008. 5(1): p. 26-36.
58. Zarzycki, M.Z. and I. Domitrz, Stimulation-induced side effects after deep brain stimulation - a systematic review. Acta Neuropsychiatr, 2020. 32(2): p. 57-64.
59. Chan, D.T., et al., Complications of deep brain stimulation: a collective review. Asian J Surg, 2009. 32(4): p. 258-63.
60. World Health Organisation. Dementia. 2023; Available from: https://www.who.int/news-room/fact-sheets/detail/dementia.
61. Assal, F., History of Dementia. Front Neurol Neurosci, 2019. 44: p. 118-126.
62. Lee, S.B., Historical sketch of dementia concept. Dement and Neurocogn Disord, 2002. 1: p. 1-2.
63. DeTure, M.A. and D.W. Dickson, The neuropathological diagnosis of Alzheimer’s disease. Molecular Neurodegeneration, 2019. 14(1): p. 32.
64. National Institute of Aging. What Happens to the Brain in Alzheimer's Disease? . 2017; Available from: https://www.nia.nih.gov/health/what-happens-brain-alzheimers-disease.
65. Animalresearch.info. Alzheimer's disease. 2022; Available from: https://www.animalresearch.info/en/medical-advances/diseases-research/alzheimers-disease/.
66. Sevigny, J., et al., The antibody aducanumab reduces Aβ plaques in Alzheimer’s disease. Nature, 2016. 537(7618): p. 50-56.
67. Gamage, K.K. and S. Kumar, Aducanumab Therapy Ameliorates Calcium Overload in a Mouse Model of Alzheimer's Disease. The Journal of Neuroscience, 2017. 37(17): p. 4430-4432.
68. Alzheimer's Society. Lecanemab: A new drug for early-stage Alzheimer’s disease 2022; Available from: https://www.alzheimers.org.uk/blog/lecanemab-new-drug-early-stage-alzheimers-disease.
69. Forny-Germano, L., et al., Alzheimer's Disease-Like Pathology Induced by Amyloid-β Oligomers in Nonhuman Primates. The Journal of Neuroscience, 2014. 34(41): p. 13629-13643.
70. Beckman, D., et al., A novel tau-based rhesus monkey model of Alzheimer's pathogenesis. Alzheimers Dement, 2021. 17(6): p. 933-945.
71. Beckman, D. and J.H. Morrison, Towards developing a rhesus monkey model of early Alzheimer's disease focusing on women's health. Am J Primatol, 2021. 83(11): p. e23289.
72. Institute, N.H.G.R. 1981-82: First Transgenic Mice and Fruit Flies. 2013; Available from: https://www.genome.gov/25520307/online-education-kit-198182-first-transgenic-mice-and-fruit-flies.
73. Stephens, D., ANDi – the first transgenic monkey. Trends in Cell Biology, 2001. 11(4): p. 151.
74. Chen, Y., et al., Modeling Rett Syndrome Using TALEN-Edited MECP2 Mutant Cynomolgus Monkeys. Cell, 2017. 169(5): p. 945-955.e10.
75. Liu, Z., et al., Autism-like behaviours and germline transmission in transgenic monkeys overexpressing MeCP2. Nature, 2016. 530(7588): p. 98-102.
76. Qiu, P., et al., BMAL1 knockout macaque monkeys display reduced sleep and psychiatric disorders. National Science Review, 2019. 6(1): p. 87-100.
77. Yang, S.H., et al., Towards a transgenic model of Huntington's disease in a non-human primate. Nature, 2008. 453(7197): p. 921-4.
78. Coors, M.E., et al., The ethics of using transgenic non-human primates to study what makes us human. Nat Rev Genet, 2010. 11(9): p. 658-62.
79. Du, Y., et al., Medical Researchers’ Ethical Awareness of Transgenic Monkeys with Human Genes: A Quantitative Case Study from China. 2021.
80. National Centre for the Replacement, R.R.o.A.i.R. The 3Rs. 2023; Available from: https://nc3rs.org.uk/who-we-are/3rs.
81. Snyder, B.R. and A.W.S. Chan, Progress in developing transgenic monkey model for Huntington's disease. J Neural Transm (Vienna), 2018. 125(3): p. 401-417.
82. Svetozarskiy, S.N., et al., [Ophthalmic manifestations of Huntington's disease]. Vestn Oftalmol, 2015. 131(5): p. 82-86.
83. Cloud, L.J., et al., Seizures in juvenile Huntington's disease: frequency and characterization in a multicenter cohort. Mov Disord, 2012. 27(14): p. 1797-800.
84. van Dijk, J.G., et al., Juvenile Huntington disease. Hum Genet, 1986. 73(3): p. 235-9.
85. Lawrence, A.D., et al., Evidence for specific cognitive deficits in preclinical Huntington's disease. Brain, 1998. 121 ( Pt 7): p. 1329-41.
86. Lawrence, A.D., et al., Executive and mnemonic functions in early Huntington's disease. Brain, 1996. 119 ( Pt 5): p. 1633-45.
87. Zizak, V.S., et al., The ubiquity of memory retrieval deficits in patients with frontal-striatal dysfunction. Cogn Behav Neurol, 2005. 18(4): p. 198-205.
88. Berrios, G.E., et al., Psychiatric symptoms and CAG repeats in neurologically asymptomatic Huntington's disease gene carriers. Psychiatry Res, 2001. 102(3): p. 217-25.
89. Davies, S.W., et al., Formation of neuronal intranuclear inclusions underlies the neurological dysfunction in mice transgenic for the HD mutation. Cell, 1997. 90(3): p. 537-48.
90. Ament, S.A., et al., High resolution time-course mapping of early transcriptomic, molecular and cellular phenotypes in Huntington's disease CAG knock-in mice across multiple genetic backgrounds. Hum Mol Genet, 2017. 26(5): p. 913-922.
91. Chan, A.W., et al., Progressive cognitive deficit, motor impairment and striatal pathology in a transgenic Huntington disease monkey model from infancy to adulthood. PLoS One, 2015. 10(5): p. e0122335.
92. Tamimi, A.F. and M. Juweid, Epidemiology and Outcome of Glioblastoma, in Glioblastoma D.V. S., Editor. 2017.
93. National Cancer Institute. Glioblastoma—Unraveling the Threads: A Q&A with Drs. Mark Gilbert and Terri Armstrong of the NIH Neuro-Oncology Branch 2017; Available from: https://www.cancer.gov/news-events/cancer-currents-blog/2017/glioblastoma-research-making-progress.
94. Desjardins, A., et al., Recurrent Glioblastoma Treated with Recombinant Poliovirus. N Engl J Med, 2018. 379(2): p. 150-161.
95. Friedman, H., et al., The Critical Role of Nonhuman Primates in Medical Research. Pathog Immun, 2017. 2(3): p. 352-365.
96. Duke Health. Poliovirus Therapy for Recurrent Glioblastoma Has 3-Year Survival Rate of 21%. 2018; Available from: https://corporate.dukehealth.org/news/poliovirus-therapy-recurrent-glioblastoma-has-3-year-survival-rate-21.
97. U.S. Food and Drug Administration. FDA Approves First COVID-19 Vaccine. 2021; Available from: https://www.fda.gov/news-events/press-announcements/fda-approves-first-covid-19-vaccine.
98. Sahin, U., K. Karikó, and Ö. Türeci, mRNA-based therapeutics — developing a new class of drugs. Nature Reviews Drug Discovery, 2014. 13(10): p. 759-780.
99. Hou, X., et al., Lipid nanoparticles for mRNA delivery. Nature Reviews Materials, 2021. 6(12): p. 1078-1094.
100. Hao, X., Monkey Research in China: Developing a Natural Resource. Cell, 2007. 129: p. 1033-6.