Bioresponsive or smart contrast agents (SCAs) are the probes capable of varying the MR image contrast upon changes in the local environment. They strongly respond to different aspects of brain physiology, such as calcium ions or amino-acid neurotransmitters, thus dramatically improving the specificity of fMRI measurements. We use a multitude of strategies to prepare these SCAs and apply them by means of the cutting-edge MRI techniques that have recently been established. After thorough physicochemical characterization, we probe their performance in vivo, aiming to visualize the neuronal signaling with high specificity and reveal important insights on brain physiology.
We are developing the following types of SCAs:
1. Calcium-sensitive contrast agents
2. Zinc-sensitive contrast agents
3. Neurotransmitter-sensitive contrast agents
Cations such as calcium(II) and zinc(II) or molecules such as neurotransmitters are essential for neuronal signaling. The ability to track their fluctuations by means of MRI would be of paramount importance for biomedical research. We therefore develop MRI sensors, molecules with integrated chelators for target cations and MR reporting moieties (Figure 1). Upon selective interaction with the cations (calcium or zinc), these SCA alternate their magnetic properties and thus the MR contrast [1-2]. Similarly, for the development of neurotransmitter-sensitive SCA, we follow the common approaches from host-guest chemistry to achieve effective molecular recognition between the host SCA and the guest amino acid neurotransmitter molecules. To this end, we employ crown-ethers as receptor molecules which interact with desired amino acids [3].
Figure 1. Our lab develops MRI-active probes responsive aspects of neural signaling, such as Ca(II), Zn(II), and amino acid neurotransmitters, which have bright outlook for the use in functional molecular imaging applications.
We take advantage of different contrast mechanisms and record the MR signals at frequencies of different nuclei to yield the maximal signal upon concentration change of the target cation or a molecule. Besides the SCAs for conventional T1- and T2-weighted MRI, we are developing sensors suitable for fluorine-based, chemical exchange saturation transfer (CEST) or hyperpolarized MRI [4-6]. To improve the biokinetic properties of these functional tracers and mitigate their use in vivo, we combine them with various nanosized and biocompatible carriers, thus obtaining multimeric, multicontrast and multimodal SCAs for molecular fMRI [7-11].
Functional MRI studies with bioresponsive contrast agents
Upon the preparation of potent SCA, we work on developing experimental procedures to validate this molecular fMRI approach in vivo and utilize its indefinite potential to visualize vasta vast number of biological processes. Our goal is establishment of routine methods that assess various aspects of neural signaling with high specificity. To this end, the above described and in-house designed and prepared small- and nano-sized SCAs are used for the development of novel functional neuroimaging methods. With these, we are pursuing the following molecular fMRI studies:
a) Dynamic fMRI recordings of extracellular calcium and amino-acid neurotransmitters. Triggering events with significant biomedical relevance are used in combination with the applied SCA to visualize various neuronal events in dynamic fashion with high spatial and temporal resolution.
b) Concentration-mapping of diverse ionic and/or molecular targets (e.g. pH or metal cations). We record multiple MRI maps in the region of SCA application by using the state-of-art MRI techniques for the quantitative assessment. This unique combination allows for the precise correlation of the recorded MRI signal with the concentration of the desired target. In turn, the quantitative molecular fMRI can substantially broaden the scope of contemporary neuroimaging techniques to study brain metabolism and function.
From the design of bioresponsive contrast agents to molecular fMRI studies
During the former period of our research, we have made remarkable progress in the development of various types of MRI contrast agents. Our expertise has been diligently developed and accumulated over the years, to result in numerous examples of bioresponsive, target-specific, multicontrast and multimodal contrast agents that have been prepared, analyzed and reported (Figure 2). We currently have the biggest library of reported Ca-sensitive MRI agents that are suitable for MRI studies at the proton and fluorine frequencies [1-2]. Together with the most recently reported Zn-sensitive probe [12], these agents exhibit the highest MRI signal changes in vitro than any other competing probes. Additionally, we have developed a series of neurotransmitter-sensitive MRI probes that respond to a range of amino acid-based neurotransmitters, such as Gly, Asp or Glu [3, 13-15].
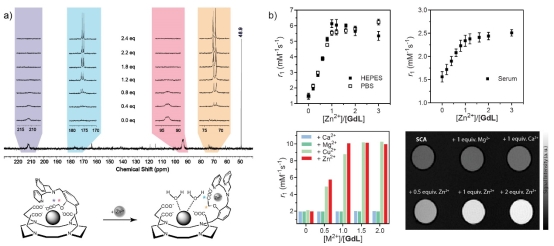
Figure 2. An example of the bioresponsive MRI agent sensitive for Zn(II), which has exhibited the best properties in vitro from all analogous probes reported in the literature to date. a) The mechanism that leads to an increase in hydration number after the probe interacts with Zn(II) (bottom), which can be confirmed by the recording a series of 13C NMR spectra (top); the broad and paramagnetically shifted signals of 13C-labeled groups (marked with asterisk) recover in intensity and return to the original chemical shift after addition of Zn(II). b) Longitudinal relaxivity changes in presence of various concentrations of Zn(II) in HEPES, PBS or human serum (top) and the effect of this interaction in presence of competing ions such as Ca(II) or Mg(II) (bottom) in two types of experiments (relaxometric and MRI – left and right, respectively). Note: the experiments presented in this figure were done with a) Eu(III) and b) Gd(III) complexes.
In parallel, we synthetically modified our bioresponsive probes and prepared liposome-, nanoparticle- or dendrimeric-based MRI agents, which showed extremely advantageous in vitro and in vivo properties [7-11]. These nanosized contrast agents display preferred biokinetic properties, i.e. have long tissue retention time, and are prone to further modifications and functional optimizations.
With these extraordinary probes in hand, we executed several studies to demonstrate their potential for functional MRI studies. Our nanoparticle-based Ca-sensitive agent was the first probe reported in the literature to show the MRI signal changes in vivo upon using calcium ions as the stimulus [7]. More recently, we also utilized a Ca-sensitive MRI probe in studying brain ischemia. We showed that an ischemic stroke can be early detected and monitored by means of our molecular fMRI approach [16]. These findings are extremely encouraging and show great potential for the development of numerous approaches to study the brain function at molecular and cellular level. Our lab will proceed in expanding the scope of the SCA-assisted fMRI to help understanding various physiological and pathological processes in brain, and consequently deliver new and valuable insights to biomedical research.
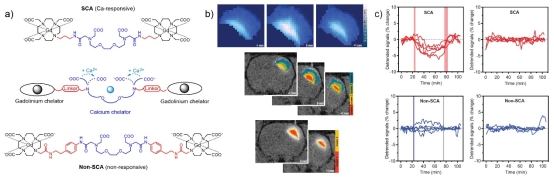
Figure 3. Our bioresponsive MRI agent sensitive for Ca(II) can detect and monitor cerebral ischemia. a) The structures of the molecules used in the functional MRI experiments: the bioresponsive probe (top), the nonresponsive-control probe (bottom) and the interaction mechanism of the bioresponsive probe with Ca(II) (middle). b) Data analysis upon the infusion of the MRI probes: coverage of the MRI probe through three MRI slices (top), as well as the K-means cluster maps (middle) or hierarchical cluster maps (bottom) of the T1-weighted signals with the bioresponsive probe. c) Detrended signals of the experiments with the responsive (left) or nonresponsive (right) probe with middle cerebral artery occlusion induction (top) and the control experiments with responsive (left) or nonresponsive (right) probe without middle cerebral artery occlusion induction.
References
[1] G. Angelovski. “Magnetic Resonance Imaging Bio-Sensors for Calcium(II)" Met. Ions Life Sci.2022, 23, 23-50. doi:10.1201/9781003229971-2. Book: Molecular Bio-Sensors and the Role of Metal Ions, Volume 23. © CRC Press, Boca Raton, USA 2022.
[2] P. Yue, G. Angelovski. “How to Develop Bioresponsive MRI Probes Based on Paramagnetic Gd(III) for in vivo Applications”. Anal. Sens.2023, 3, e202300019.
[3] G. Angelovski, É. Tóth. “Strategies for Sensing Neurotransmitters with Responsive MRI Contrast Agents.” Chem. Soc. Rev., 2017, 46, 324-336.
[4] G. Gambino, T. Gambino, R. Pohmann, G. Angelovski. “A ratiometric 19F MR-based method for quantification of Ca2+ using responsive paramagnetic probes”. Chem. Commun.2020, 56, 3492-3495.
[5] T. Gambino, L. Valencia, P. Pérez-Lourido, D. Esteban-Gómez, M. Zaiss, C. Platas-Iglesias and G. Angelovski. “Inert Macrocyclic Eu3+ Complex with Affirmative paraCEST Features”. Inorg.Chem. Front.2020, 7, 2274-2286.
[6] G. Angelovski, B. J. Tickner, G. Wang. “Opportunities and challenges with hyperpolarized bioresponsive probes for functional imaging using magnetic resonance”. Nature Chem.2023, 15, 755-763.
[7] A. Moussaron, S. Vibhute, A. Bianchi, S. Gündüz, S. Kotb, L. Sancey, V. Motto-Ros, Y. Crémillieux, F. Lux, N. K. Logothetis, O. Tillement, G. Angelovski. “Ultra-small Nano-platforms as Calcium-Responsive Contrast Agents for Magnetic Resonance Imaging.” Small, 2015, 11, 4900-4909.
[8] S. Gündüz, N. Nitta, S. Vibhute, S. Shibata, M. E. Maier, N. K. Logothetis, I. Aoki, G. Angelovski. „Dendrimeric Calcium-responsive MRI Contrast Agents with Slow in vivo Diffusion.” Chem. Commun.2015, 51, 2782-2785.
[9] F. Garello, S. Vibhute, S. Gündüz, N. K. Logothetis, E. Terreno, G. Angelovski. “Innovative Design of Ca-Sensitive Paramagnetic Liposomes Results in an Unprecedented Increase in Longitudinal Relaxivity”, Biomacromolecules2016, 17, 1303-1311.
[10] S. Gündüz, T. Savić, R. Pohmann, N. K. Logothetis, K. Scheffler, G. Angelovski. “Ratiometric Bioresponsive MRI Contrast Agent for Rapid Monitoring of Biological Processes”, ACS Sens.2016, 1, 483-487.
[11] G. Gambino, T. Gambino, G. Angelovski. “Combination of Bioresponsive Chelates and Perfluorinated Lipid Nanoparticles Enables in vivo MRI Probe Quantification”. Chem. Commun.2020, 56, 9433-9436.
[12] G. Wang, G. Angelovski. “Highly Potent MRI Contrast Agent Displaying Outstanding Sensitivity to Zinc Ions”. Angew. Chem. Int. Ed.2021, 60, 5734-5738,
[13] F. Oukhatar, S. Même, W. Même, F. Szeremeta, N. K. Logothetis, G. Angelovski, É. Tóth. “MRI sensing of neurotransmitters with a crown-ether appended Gd3+ complex.” ACS Chem. Neurosci.2015, 6, 219-225.
[14] Đ. Toljić, C. Platas-Iglesias, G. Angelovski. “In-depth study of a novel class of ditopic gadolinium(III)-based MRI probes sensitive to zwitterionic neurotransmitters”. Front. Chem.2019, 7, 490.
[15] Đ. Toljić, G. Angelovski. “A low-molecular weight ditopic MRI probe for ratiometric sensing of zwitterionic amino acid neurotransmitters”. Chem. Commun.2019, 55,11924-11927.
[16] T. Savić, G. Gambino, V. S. Bokharaie, H. R. Noori, N. K. Logothetis, G. Angelovski. “Early detection and monitoring of cerebral ischemia using calcium-responsive MRI probes”. Proc. Natl. Acad. Sci. U.S.A.2019, 116, 20666-20671.